
A research team led by Prof. LIU Chengbo, Prof. YAN Fei, and Prof. CHU Jun from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences, in collaboration with Prof. Jonathan Sessler from The University of Texas at Austin, has proposed a new approach to achieve background-suppressed tumor-targeted photoacoustic imaging.
Their study was published in PNAS on Feb. 23.
Photoacoustic (PA) imaging is promising for biomedical applications due to its ability to image deep within biological tissues while providing detailed molecular information; however, its detection sensitivity is limited by high background signals that arise from endogenous chromophores such as hemoglobin and melanin.
Genetic reporter proteins with photoswitchable properties can remove background signals through the subtraction of PA images for each light-absorbing form.
Unfortunately, the application of photoswitchable chromoproteins for targeted imaging has been hampered by the lack of an effective targeted delivery mechanism. In other words, photoswitchable probes need to be delivered in vivo with high targeting efficiency and specificity.
In this study, the researchers proposed an approach called GPS localization. In this approach, "G" stands for genetically encoded probe, "P" for photoacoustic imaging, and "S" for synthetic biology.
The genetically encoded probe is a newly engineered photoswitchable chromoprotein (F469W). Its efficiency in eliminating blood background signals is the highest reported so far.
The photoacoustic imaging method uses a house-made visualization tool to achieve deep-tissue tumor-specific imaging in vivo.
As for synthetic biology, a bacterial vector (E. coli) has been reprogrammed to produce the photoswitchable chromoprotein (F469w) and deliver it to the tumor region. Owing to their anaerobic features, the F469w-expressing E. coli show tumor-preferential colonization ability; thus, genetically encoded probes can be delivered to the microenvironment of the tumor site.
This approach is capable of achieving tumor-targeted imaging while eliminating interference from blood background signals.
In contrast to conventional molecular probes which are easily diluted and cleared in vivo, E. coli is able to colonize cancerous regions while producing the F469w chromoprotein, thus facilitating long-term, stable tumor imagining.
Moreover, E. coli can be effectively removed after drug (e.g., streptomycin) intervention. Therefore, this E. coli-based targeting strategy can be well controlled.
In the future, GPS imaging may help with the initial diagnosis and long-term monitoring of tumor-related diseases.
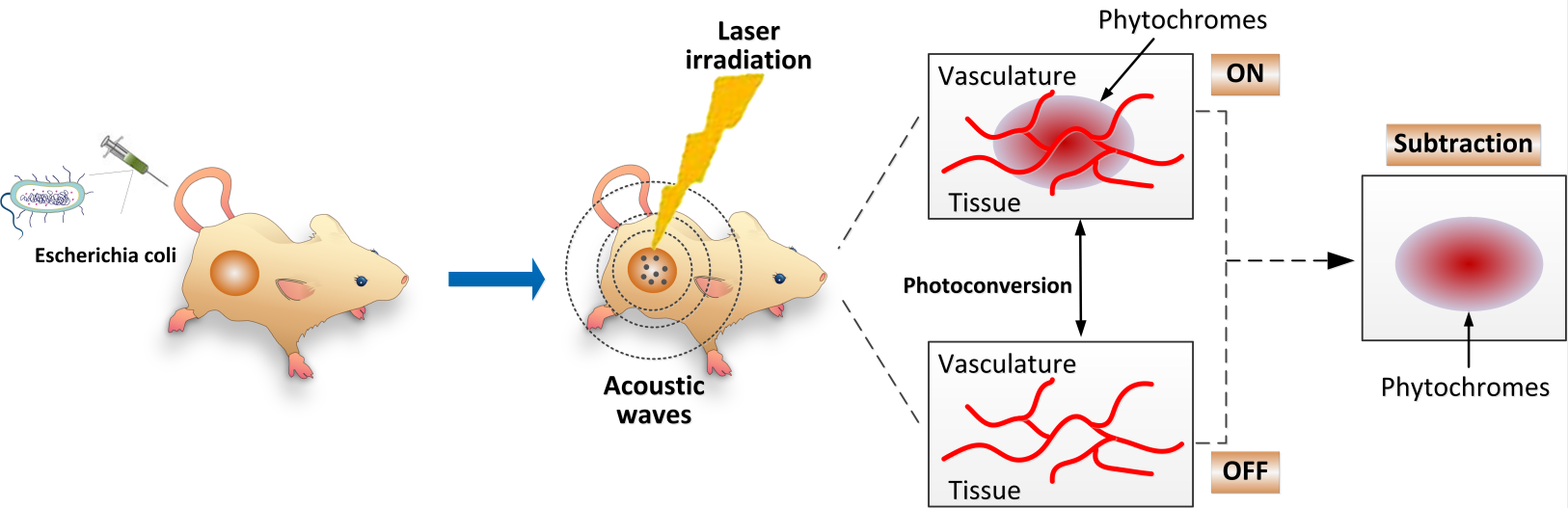
Schematic diagram of the bacteria-based in vivo delivery system for photoswitchable chromoproteins. (Image by SIAT)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

