
Newsroom
What neural and behavioral mechanisms allow mice to identify food sources, navigate toward them, and initiate consumption? A research team led by Prof. ZHANG Yunfeng from the Institute of Zoology of the Chinese Academy of Sciences has uncovered the neural encoding mechanisms that enable mice to use their olfactory system to assess prey nutritional status and make precise foraging decisions at the molecular, cellular, and neural circuit levels.
The study was recently published in Proceedings of the National Academy of Sciences (PNAS).
In natural environments, animals often rely on odors to evaluate food nutritional value. Odor molecules from food sources provide predators with critical information about nutritional quality and palatability. For mice, the ability to assess food nutritional status via olfactory cues likely determines whether they start feeding behavior. However, the underlying neural mechanisms have remained unclear.
To address this gap, the researchers developed a behavioral experimental system simulating natural predation, using cotton bollworm larvae as the prey for mice. Notably, whether mice were fasted or sated, they all showed a significant preference for fed larvae over unfed ones, and the main olfactory system proved indispensable for this process.
Using gas chromatography–mass spectrometry, the team precisely identified two key chemical compounds in the larvae's surface volatiles: linoleic acid (LA), which was more abundant on well-fed larvae, and (Z)-9-tricosene [(Z)-9-TE], which was concentrated on the surface of unfed larvae.
Further investigation revealed that LA attracted mice, while (Z)-9-TE triggered avoidance behavior, with both responses showing dose dependence. Additional studies identified the dopaminergic neural pathway originating from the ventral tegmental area (VTA), and projecting to the medial olfactory tubercle (mOT) as a critical hub for regulating odor preference.
In vivo fiber photometry and pharmacological experiments showed that D1- and D2-type medium spiny projection neurons in the mOT responded specifically to LA and (Z)-9-TE, respectively. Specifically, D1 receptor signaling mediated attraction to LA, while D2 receptor signaling was involved in avoidance of (Z)-9-TE. These two pathways form a finely balanced "seesaw"-like model that coordinates mice's predation of larvae.
This study enhances understanding of ecological species interactions and offers potential for developing pest management strategies by targeting conserved olfactory evaluation pathways.
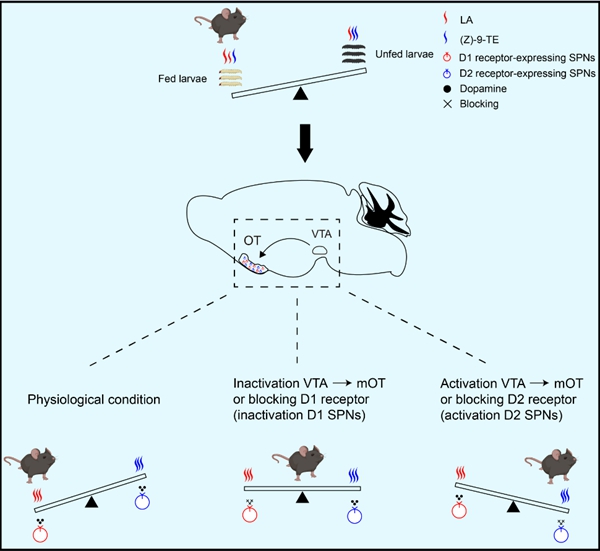
Schematic demonstrating a "seesaw" working model of the VTA-mOT dopaminergic pathway in orchestrating preference for fed over unfed larvae. (Image by Prof. ZHANG Yunfeng's group)
