
Newsroom
Cells operate like miniature cities, with kinesin motor proteins serving as vital couriers transporting intracellular cargo along microtubule "highways." Among these, kinesin-2 is a specialized subclass of kinesins. The canonical kinesin-2 forms a stable heterotrimeric complex composed of two distinct motor subunits (e.g., KIF3A/KIF3B or KIF3A/KIF3C) and one non-motor subunit, KAP3. This heteromeric configuration is essential for specialized processes such as anterograde transport in cilia. However, the mechanisms of its assembly have remained elusive.
On July 24, a research team led by Prof. FENG Wei from the Institute of Biophysics of the Chinese Academy of Sciences published a study in Nature Communications detailing the structural and functional basis of kinesin-2 assembly.
Focusing on the heterotrimeric kinesin-2 complex from Caenorhabditis elegans comprising KLP-11, KLP-20, and KAP-1, the researchers combined structural biology with in vivo functional analyses to elucidate the mutual co-recognition mechanisms underlying complex formation and its physiological relevance.
They first reconstituted the heterotrimeric kinesin-2 complex by co-expressing its components in vitro and determined its overall architecture using single-particle cryo-electron microscopy. This structural framework guided further protein optimization, culminating in a 3.5 Å resolution crystal structure resolved through X-ray crystallography.
Their analysis revealed that the tails of the two motor subunits intertwine via conserved hetero-pairing trigger sequences (HTSs), forming a heterodimer that cooperatively engages the C-terminal helical hook (CTH-hook) of KAP-1. Simultaneously, the central target-binding groove of KAP-1 anchors both motor tails, stabilizing the entire complex. This arrangement illustrates a precise and robust mode of assembly based on mutual co-recognition among the three subunits.
To assess the functional relevance of these structural features, the researchers introduced targeted mutations into key interface residues and examined their impact on complex formation using co-immunoprecipitation assays. These experiments confirmed that the identified interfaces are critical for kinesin-2 assembly.
They then extended to live C. elegans, where the researchers expressed the mutants in sensory neuron cilia and visualized intraflagellar transport (IFT) dynamics using fluorescently labeled IFT proteins. These in vivo analyses further validated that mutual co-recognition is essential for efficient ciliary transport.
Interestingly, the interface regions of the kinesin-2 complex contain multiple putative phosphorylation sites. Phosphorylation at these positions may destabilize the interaction interfaces and promote complex dissociation, suggesting a regulatory mechanism that allows dynamic cycling of the kinesin-2 complex during ciliary transport.
"Mutations in genes such as KIF3A and KIF3B are associated with a range of ciliopathies, including retinitis pigmentosa, polycystic kidney disease, and neurodevelopmental disorders," said Prof. FENG. "Understanding how kinesin-2 assembles offers insights into the molecular basis of these diseases and opens potential avenues for targeted therapies and gene-based interventions."
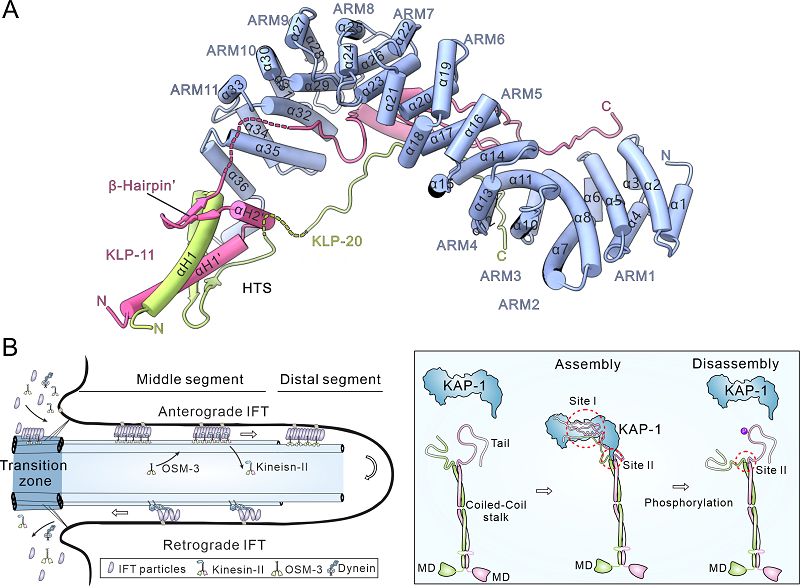
Overall structure of the tail region of the heterotrimeric kinesin-2 motor and schematic model of its role in regulating intraflagellar transport (IFT) (Image by FENG Wei's group)
