
Newsroom
Mitochondria play a crucial role in acquiring pluripotency and determining cell fate. The mitochondrial unfolded protein response (UPRmt) serves as an important retrograde regulatory pathway that communicates from mitochondria to the nucleus. Various factors, including imbalances in mitochondrial protein homeostasis, elevated reactive oxygen species (ROS), discrepancies in mitochondrial and nuclear gene expression, and mitochondrial dysfunction, can all activate UPRmt. The activation of this pathway regulates gene expression, thereby influencing cellular physiological or pathological processes.
In nematodes, UPRmt has been shown to delay aging, and its regulatory and activation mechanisms are relatively well understood. However, in mammalian cells, the regulatory mechanisms of UPRmt are more complex, and its role in cell fate determination and development has not been extensively reported.
To address this challenge, a research team led by Prof. LIU Xingguo from the Guangzhou Institutes of Biomedicine and Health of the Chinese Academy of Sciences has published a study in Nature Metabolism. They discovered that UPRmt is transiently activated during the early stages of acquiring pluripotency and gradually diminishes thereafter. Also, they identified c-Myc as a key factor in the activation of UPRmt; overexpression of c-Myc significantly increased the expression of Hsp60, a marker of UPRmt, while other transcription factors (such as Sox2, Klf4, and Oct4) did not exhibit similar effects.
Further research revealed that the activation of UPRmt during pluripotency acquisition inhibits the mesenchymal-to-epithelial transition (MET). Notably, UPRmt also plays a regulatory role in MET during early mammalian differentiation and in tumors. The activation of UPRmt can enhance the migration and invasion capabilities of cancer cells.
In exploring the underlying mechanisms, the team identified c-Jun, a novel key factor in the UPRmt retrograde signaling pathway. c-Jun, a proto-oncogene, inhibits the acquisition of pluripotency, and the activation of UPRmt promotes c-Jun expression. Through transcriptional regulation, c-Jun upregulates the expression of acetyl-CoA metabolic enzymes, thereby reducing acetyl-CoA levels. Since acetyl-CoA is closely associated with histone acetylation, the researchers discovered that UPRmt activation decreases histone acetylation, particularly the level of H3K9 acetylation (H3K9Ac).
The study also found that activated UPRmt reduces the binding of H3K9Ac to the promoter regions of epithelial-related genes (such as E-cadherin and Epcam). This reduction in H3K9Ac levels leads to decreased expression of these epithelial-related genes, ultimately suppressing MET. By supplementing precursors of acetyl-CoA (such as acetate, citrate, and pyruvate), THE researchers were able to restore H3K9Ac levels, along with the expression of MET-related genes.
In summary, this study is the first to reveal the molecular mechanism by which UPRmt, through c-Jun, reduces acetyl-CoA levels and decreases histone acetylation, inhibiting MET and hindering the acquisition of pluripotency. It highlights how mitochondrial pathways regulate MET and suggests that this regulatory mode exists in early differentiation and tumor contexts, indicating a broad biological mechanism. This discovery offers new insights into how mitochondria retroactively regulate the nucleus and influence cell fate determination, potentially paving the way for advancements in regenerative medicine and new targets for cancer therapy.
This study was conducted in collaboration with several research groups, including Guangzhou Medical University, the Innovation Center for Regenerative Medicine and Health at the Hong Kong Institute of Innovation under the Chinese Academy of Sciences, and The Chinese University of Hong Kong.
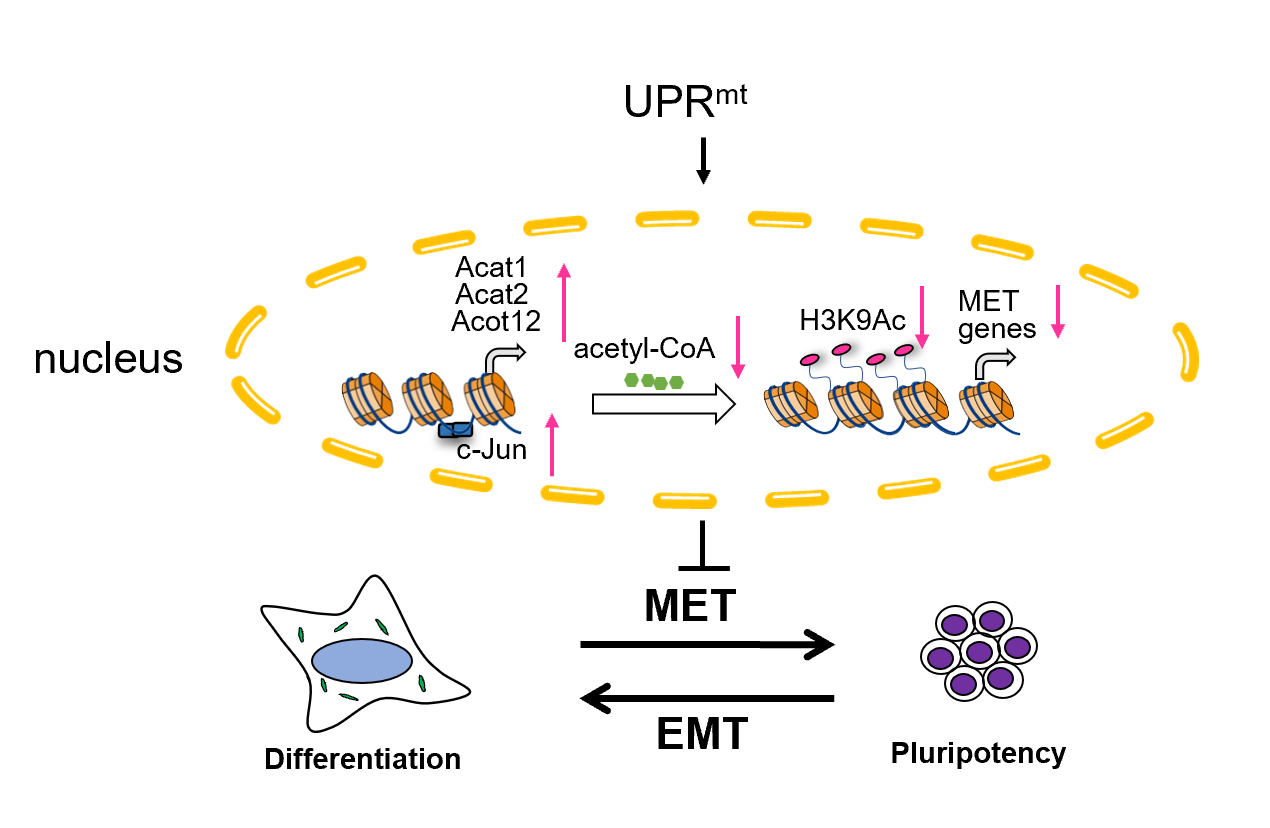
Scheme showing the role of activated UPRmt during the early phase of reprogramming. (Image by Prof. LIU's team)
