
Newsroom
Enzymes, the core catalysts in life, drive critical biological processes ranaging from metabolic regulation to energy conversion. Evolved over billions of years, these versatile molecular machines not only serve as foundational elements in biological systems but also offer pivotal tools in synthetic biology, transcending the limitations of traditional chemical synthesis. Acting as micro-factories, enzymes enable the efficient production of antibiotics, biofuels, high-value compounds, and other desired products.
In a recent study, researchers from the Tianjin Institute of Industrial Biotechnology of the Chinese Academy of Sciences (TIBCAS), together with collaborators from Hangzhou Normal University, have achieved a breakthrough in deciphering enzymatic mechanism. In the study published in Nature, they revealed the catalytic role of reactive oxygen species (ROS) superoxide (O₂•⁻) in a haem-catalase mediated synthesis of ergot alkaloids (EAs), a group of medicinal natural products for treating various diseases.
The TIBCAS team discovered a unique enzymatic mechanism involving the haem-catalase enzyme EasC, which plays a key role in EA biosynthesis. They found that EasC contains two distinct catalytic "workshops": one located within the enzyme's haem pocket, and another on its surface pocket, connected by a slender tunnel. The internal workshop generates superoxide, which is then transported via the tunnel to the surface workshop, where it catalyzes a series of radical reactions that convert substrates into the final EA products.
This "dual-workshop with a transport pipeline" enzymatic mechanism is akin to constructing two specialized facilities on a molecular scale—one producing ROS and the other synthesizing EA—while establishing a dedicated transport tunnel for ROS. This spatial segregation and transport strategy harnesses the potent reactivity of ROS while circumventing its destructive potential, showcasing the evolutionary ingenuity of microbial enzyme systems in oxygen chemistry.
Remarkably, the study found that the reduction of O2 for ROS production in the haem pocket, traditionally thought to require external electron donors, is instead directly powered by the substrate here.
While superoxide is typically recognized for its destructive effects on DNA, proteins, and other cellular molecules, this study highlights a novel, constructive role for the molecule in biosynthesis. It underscores nature's evolutionary ingenuity, revealing that ROS can be strategically employed as catalytic agents in complex biochemical pathways.
The implications of this research extend beyond the laboratory. In 2024, lysergic acid diethylamide (LSD), a semi-synthetic EA, received breakthrough therapy designation from the FDA for generalized anxiety disorder, further underscoring the EA's clinical importance. The new insights from this study could accelerate the development of cell factories for the sustainable production of ergot alkaloids and provide a molecular blueprint for designing novel enzymes. This could lead to greener, low-carbon alternatives to traditional chemical synthesis, marking a shift toward more efficient and eco-friendly pharmaceutical manufacturing.
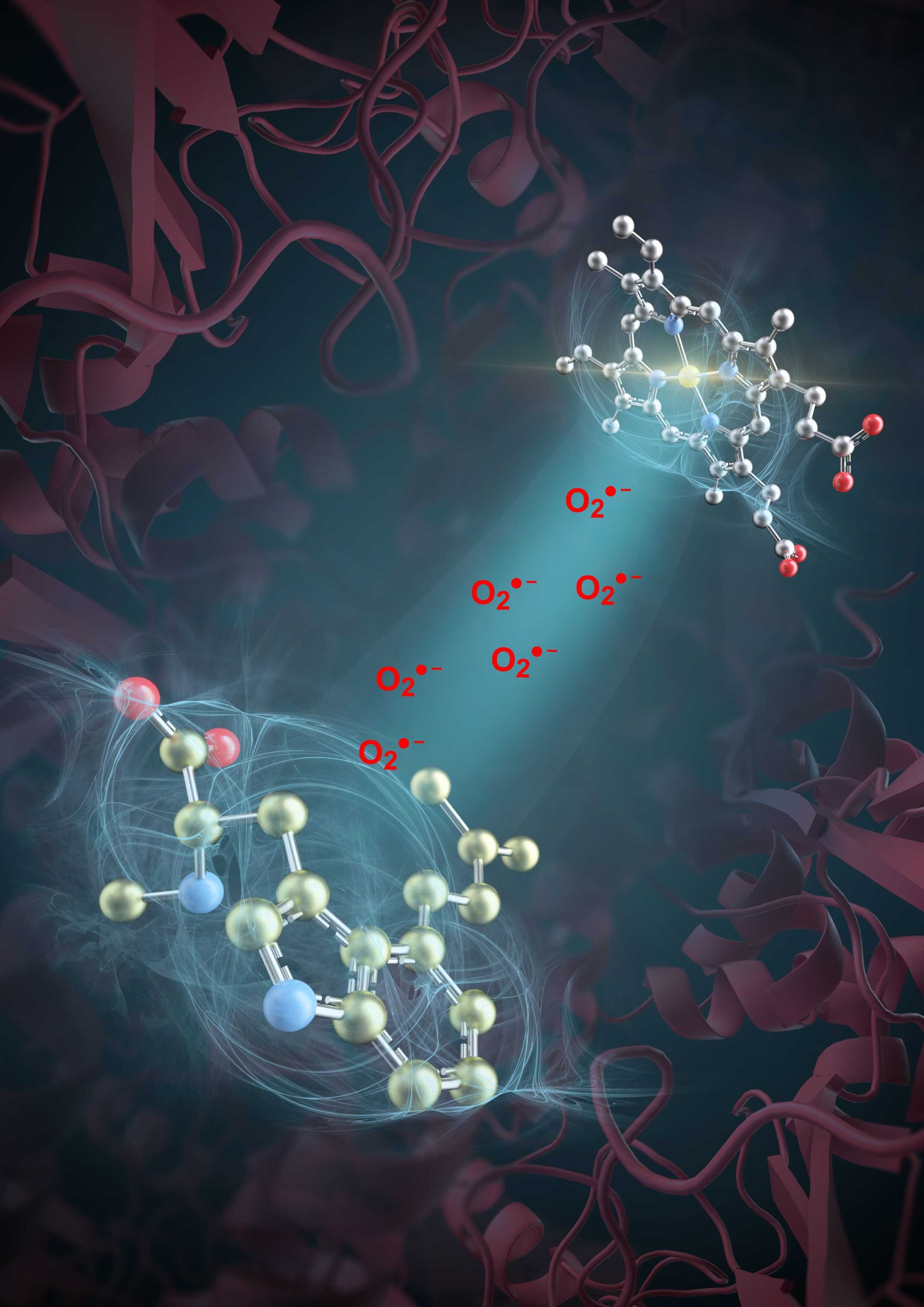
Schematic diagram of catalase generation of superoxide anion catalyzed ergot line natural drug molecule biosynthesis ( Image by TIBCAS)
