
Pyroptosis is a type of programmed cell death mediated by the gasdermin (GSDM) protein family, which plays important roles in the body's defense against pathogen infection, elimination of abnormal or harmful cells, and other processes. GSDMs are an evolutionarily conserved class of pore-forming proteins that is widely distributed among various bacteria, fungi, invertebrates, and all vertebrates. GSDMs typically have an autoinhibited two-domain structure. Proteolytic cleavage appears to be a universal mechanism for the activation of all GSDMs. Whether there are activation mechanisms other than protease cleavage for GSDMs is unknown.
In a study published in Science on April 25, researchers led by Prof. DING Jingjin from the Institute of Biophysics of the Chinese Academy of Sciences and Prof. SHAO Feng from the National Institute of Biological Sciences have revealed novel mechanisms for cleavage-independent activation of two types of GSDM proteins from lower eukaryotes.
Through extensive sequence homology analysis, the researchers first identified a GSDM protein in the basal metazoan Trichoplax adhaerens (TrichoGSDM) that contains only a pore-forming domain. Characterization of purified TrichoGSDM revealed that this GSDM protein exists in two states: monomer and homodimer, with only the monomeric protein having the ability to form pores on liposomes.
Structural and biochemical analyses revealed that the disulfide bonded homodimer represents the autoinhibitory state of TrichoGSDM, which is activated to the monomeric state by reducing disulfide bonds, further oligomerizing and forming pores on the cell membrane to induce pyroptosis-like cell death. This novel activation mechanism, discovered in TrichoGSDM, is the first of its kind in the entire GSDM family.
In addition, the researchers focused on another type of pore-forming domain-only GSDM protein called RCD-1, which was newly identified in the filamentous fungus Neurospora crassa. RCD-1 contains two homologous proteins, RCD-1-1 and RCD-1-2 in different strains, governing allorecognition-induced fungal cell death.
They found that membrane-bound RCD-1 proteins exist in an inactive resting state when left alone. However, when different strains undergo cell fusion, the two RCD-1 proteins encounter each other and assemble into a heterodimer through specific intermolecular recognition, further forming heterooligomeric pores on the cell membrane to execute pyroptosis-like cell death.
In this study, TrichoGSDM and RCD-1 represent two types of pore-forming domain-only GSDMs derived from simple and ancient eukaryotes that use distinct cleavage-independent activation mechanisms. TrichoGSDM is a disulfide-linked autoinhibited dimer and is activated by reduction of the disulfides, suggesting a redox-responsive function. The pore-forming activity in RCD-1 is stimulated by hetero-recognition between RCD-1-1 and RCD-1-2 from genetically incomparable fungal strains, underlying allorecognition-induced cell death in N. crassa.
The diverse activation mechanisms suggest that GSDM proteins can respond to a wide range of physiological signals and participate in multiple biological processes. Furthermore, these pore-forming domain-only GSDM proteins have the potential to be developed as novel tools to induce cell death independent of protease cleavage, facilitating pyroptosis-related basic and translational research.
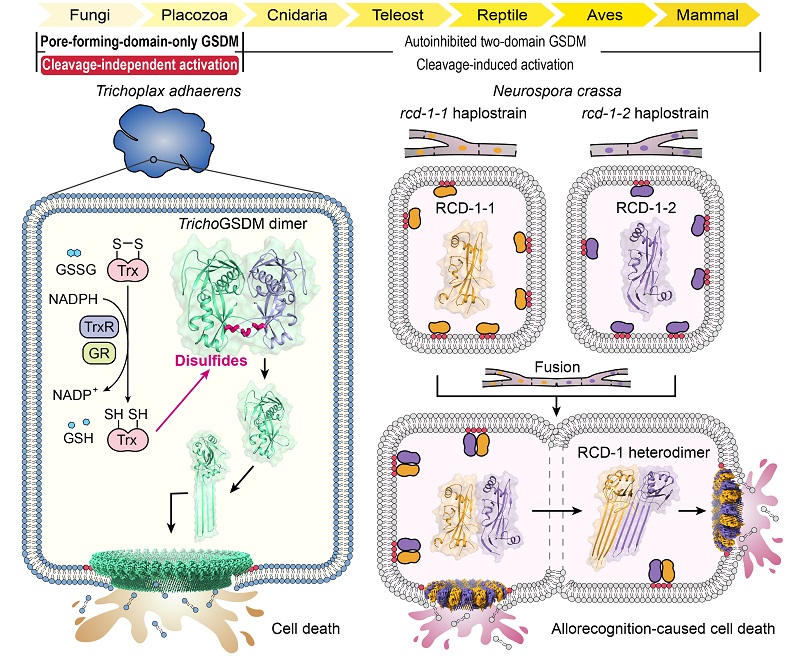
Basal eukaryotes Trichoplax adhaerens and Neurospora crassa both harbor pore-forming-domain-only GSDMs that are activated to cause cell death through cleavage-independent mechanisms. (Image by DING Jingjin's group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

