
Newsroom
Endoplasmic reticulum (ER) is the folding factory of secretory and membrane proteins in eukaryotic cells. Changes in the internal and external environment of the cell cause imbalance of ER homeostasis including proteostasis, redox homeostasis and calcium homeostasis. When the protein folding burden exceeds the folding ability in the ER, ER stress occurs. Three transmembrane “sensor” proteins including IRE1 on the ER membrane then initiate a series of signal transduction from the ER to the nucleus. These cellular events are collectively referred to as unfolded protein response (UPR).
The inherent latency of the UPR limits its responsiveness to changes in the burden of unfolded proteins. In particular, dedicated secretory cells experience recurring physiological fluctuations in the unfolded protein load that appear too transient for alterations of the transcriptional program alone to handle, hinting at the need for other compensatory mechanisms to adjust the folding capacity.
A recent study published online in The EMBO Journal and by Prof. WANG Chih-chen’s group from Institute of Biophysics of the Chinese Academy of Sciences reported that during early endoplasmic reticulum ER stress, the secretory pathway kinase Fam20C catalyzes the phosphorylation of protein disulfide isomerase (PDI) to regulate its functional switch, which is critical for maintaining ER proteostasis and attenuating ER stress.
The secretory pathway kinase Fam20C has been revealed in the previous study to tune ER redox homeostasis via phosphorylation of ER sulfhydryl oxidaseEro1α, which plays an important role in hypoxia stress, reductive stress and lactation.
In this study, the researchers first found that Fam20C depletion sensitizes the IRE1α branch of the UPR under ER stress. Through unbiased proteomics analysis, they then identified that the phosphorylation of PDI, one of the most abundant and critical folding catalysts in the ER, as an early event during ER stress.
Fam20C phosphorylates Ser357 in the x-linker of PDI and responds rapidly to various ER stressors. Phosphorylation of Ser357 induces an open conformation of PDI and turns it from a ‘foldase’ to a ‘holdase’, which is critical for preventing protein misfolding in the ER. The amplitude and duration of IRE1α activation determines cell fate under ER stress, and the precise control of IRE1α activity is of vital importance.
Therefore, the researchers showed that phosphorylated PDI binds to the luminal domain of IRE1α, and attenuates its excessive activity. Importantly, PDI S359A knock-in mice display enhanced IRE1α activation, elevated levels of proinflammatory and proapoptotic factors and liver damage under acute ER stress.
The finding uncovers a novel mechanism for how cells rapidly and sensitively respond to ER stress through phosphorylation of PDI by Fam20C to maintain ER proteostasis and protect against ER stress-induced cell death. It also reveals that PDI is a new ER stress-activated molecular chaperone, perfectly interpreting and enriching Prof. WANG Chih-chen and Prof. TSOU Chen-lu’s scientific assertion about "PDI is both an enzyme and a molecular chaperone" proposed in the 1990s.
This study not only expands the understanding of ER stress, but also provides a new biomarker and possible interventions to combat ER stress-associated diseases.
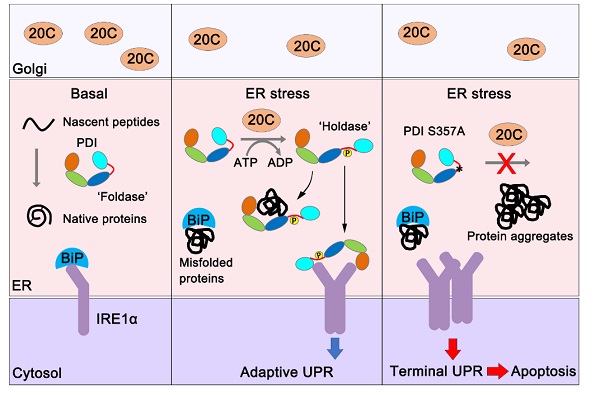
Fam20C-catalyzed phosphorylation switches protein disulfide isomerase activity to maintain proteostasis and attenuate ER stress. (Image by Prof. WANG Chih-chen’s lab)
