
Endoplasmic Reticulum-Associated Degradation (ERAD) is a special ubiquitin proteasome system located on endoplasmic reticulum (ER) and is responsible for removing misfolded proteins retained in the ER. Researches in yeast and mammals showed that ERAD has functions in stress- and disease-resistant and also participates in plant stress response. However, the detail mechanism underlies the function of ERAD in stress response in plant is unclear.
Actually, there is a small amount of misfolded proteins under standard growth condition and thus ERAD’s function should maintain in a lower activity state by removing some key ERAD components, which is termed as ERAD tuning. However, to date there is no report on ERAD tuning in plant.
Researchers from Dr. XIE Qi’s group at the Institute of Genetics and Developmental Biology (IGDB) of Chinese Academy of Sciences elucidated the key molecular mechanism of ERAD tuning in plant. This study was published online in Nature Plants with graduate student CHEN Qian as the first author.
The previous studies revealed that the plant ubiquitin-conjugating enzyme 32 (UBC32) and HMG-COA reductase degradation 3A (HRD3A) are ERAD components in degradation of alpha2 10 (DOA10) and HRD1 complexes respectively.
Based on their knowledge of HRD3A and UBC32, researchers from XIE’s group investigated the relationship between these two ERAD complexes. They found that plant UBC32, working in the DOA10 complex, is maintained at low levels under standard conditions by proteasome-dependent degradation mediated by the HRD1 complex, the other E3 complex involved in ERAD.
They then found that loss of this negative regulation under ER stress increases capacity for degradation of misfolded proteins retained in ER.
Extend this work to mammal, they found that UBE2J1, the homolog of UBC32 in mammals, is also targeted by HRD1 for degradation. This result challenges the knowledge in the ERAD field for the last decade.
Their results revealed that the regulation of UBC32 (or UBE2J1) by HRD1 complex is conserved between plants and mammals.
This work was supported by grants from the Ministry of Science and Technology and the National Science Foundation of China.
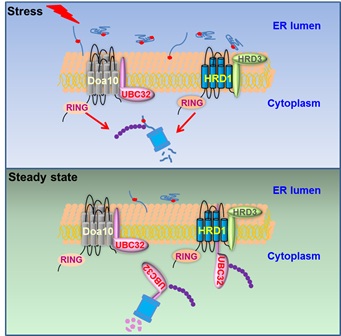
Figure: The proposed working model of ERAD tuning in plants and mammals (Image by IGDB)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

