
Newsroom
Mature or nearly mature fruits of Piper longum are used as a spice, valued for their commercial and industrial applications, as well as in traditional Chinese medicine for their multiple effects, such as dispelling cold and relieving pain. Given their long history of medicinal use, the fruits of P. longum present an opportunity to explore their therapeutic constituents. However, the chemical components of traditional Chinese medicines are often complex, making the efficient discovery of novel active compounds a challenging task in natural product research.
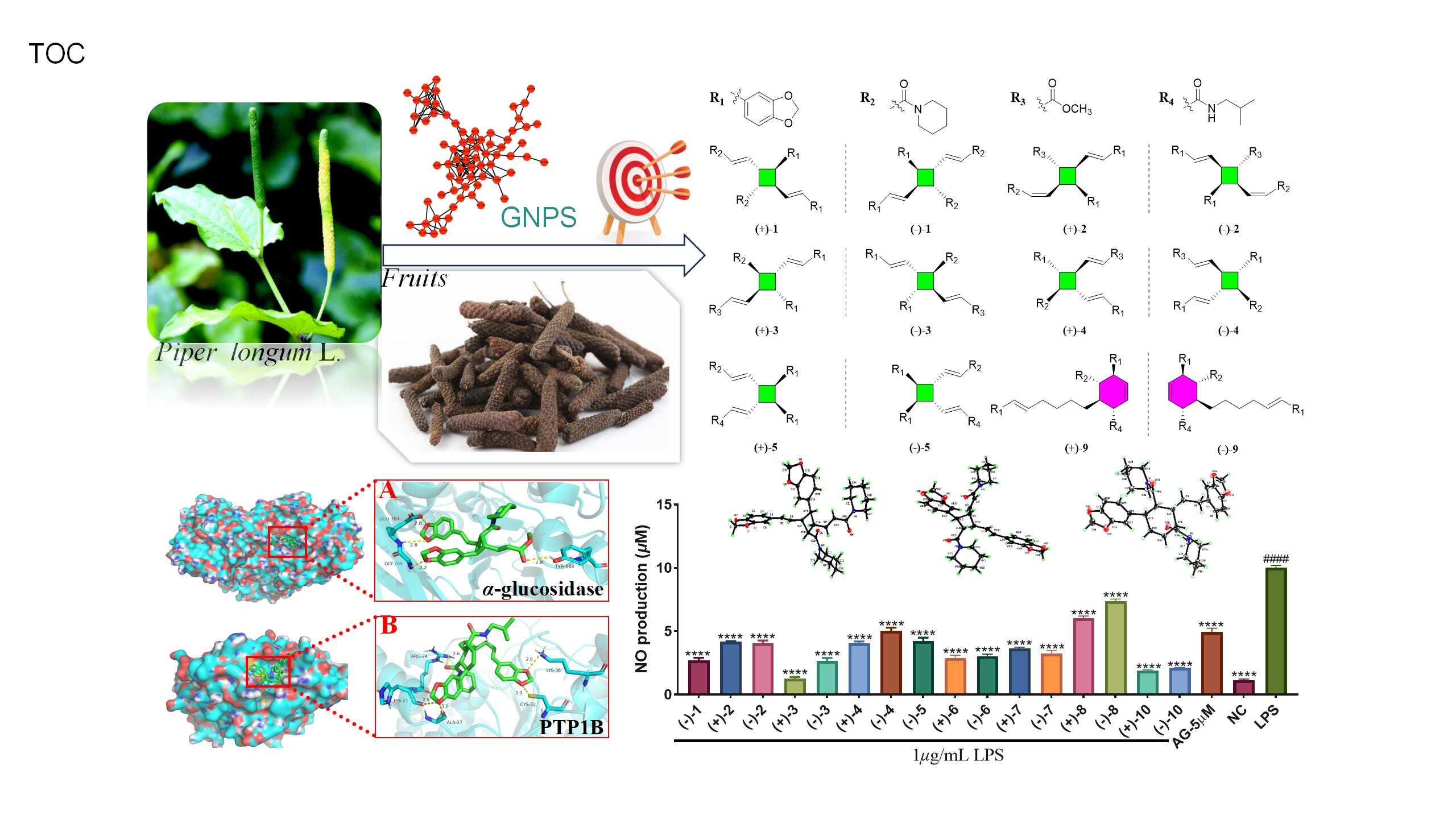
To address this challenge, a research team led by Prof. Haji Akber Aisa from the Xinjiang Technical Institute of Physics & Chemistry of the Chinese Academy of Sciences isolated 12 dimeric amide alkaloid enantiomers with anti-inflammatory and antidiabetic effects from P. longum fruits using a molecular network-based dereplication strategy. This study was published in the Journal of Agricultural and Food Chemistry.
The researchers created a molecular network of a 95% ethanol extract of P. longum fruits using the GNPS platform's Feature-Based Molecular Networking (FBMN) module. A distinct cluster was identified within the network, which included nodes with molecular weights ranging from 486.191 to 653.255 Da, but which did not correspond to known chemical structures in public databases. A specific node in this cluster, with a molecular weight of 627.3420 Da and corresponding to a previously identified amide alkaloid dimer from P. longum, named piperchabamide H (m/z 627.3428 [M + H]+), served as a "seed molecule", leading to the isolation of 12 dimeric amide alkaloids. The researchers successfully separated these 12 dimeric amide alkaloids into 12 pairs of enantiomers using chiral HPLC.
The complete structures of these compounds were elucidated through comprehensive spectroscopic data, electronic circular dichroism (ECD) calculations, and X-ray diffraction analysis. The identified dimeric amide alkaloids included eight pairs of cyclobutane-type dimers and four pairs of cyclohexene-type dimers, featuring five pairs of new cyclobutane-type dimers and one pair of a new cyclohexene-type dimer.
In vitro bioactivity screening revealed that three compounds exhibited notable anti-inflammatory effects in an LPS-induced RAW 264.7 macrophage model. Additionally, one compound demonstrated significant inhibitory activity against α-glucosidase, while three compounds showed promising inhibitory activity against protein tyrosine phosphatase-1B (PTP1B).
To investigate the interaction mechanisms between these dimeric amide alkaloids and the enzymes α-glucosidase and PTP1B, molecular docking studies were conducted using the two most active compounds as representatives. The results indicated that both compounds formed stable complexes with their respective target proteins. Further molecular dynamics simulations provided additional evidence supporting the stability of the complexes formed between the active compounds and the corresponding proteins.
This study highlights the potential application of these amide alkaloid dimers in developing functional foods and pharmaceutical products, thereby extending the health-promoting benefits of P. longum fruits.
This work was supported by the Tianshan Talent Training Program of the Science & Technology Department of the Xinjiang Uygur Autonomous Region, the Natural Science Foundation of China, and the National Key R&D Program of China.
Discovery of dimeric amide alkaloid enantiomers by a molecular network-based dereplication strategy from Piper longum fruits and their bioactivities (Image by Prof. Haji Akber Aisa’s team)
