
Researchers led by Prof. LIU Jianjun from the Shanghai Institute of Ceramics of the Chinese Academy of Sciences, cooperating with Prof. TANG Weiping's group from the Shanghai Institute of Space Power-Sources, have recently designed a high-performance solid electrolyte of Li3Zr2Si2PO12 (LZSP) with space group of C12/c1 (No. 15) from the Na3Zr2Si2PO12 (NZSP) precursor through a skeleton-retained cationic exchange approach.
The researchers have proposed an experimental and theoretical strategy to enhance the conductivity of solid electrolyte ions by relocating diffusion ions to under-coordination sites, which opens up an innovative way for the production of new materials. Results were published in Science Advances.
Solid electrolytes are the important materials for improving safety, energy density and reversibility of electrochemical energy storage batteries. Although some sulfide solid electrolytes, such as Li10GeP2S12, perform high ionic conductivity, but the low stability against Li metal leads to a fast decrease of ionic conductivity.
Other solid electrolytes, such as oxide solid electrolyte with overall stability (electrochemical, chemical and environmental), show low ionic conductivity, hampering the further application, which essentially due to the narrow bottleneck of lithium ion from octahedral to tetrahedral occupying. Therefore, achieving a high ionic conductivity for oxide solid electrolyte has been a long-term and difficult task due to the limit of the bottleneck size.
In this study, the researchers proposed a strategy of skeleton-retained cationic exchange (Na+ → Li+) to synthesize LZSP material in experiment and revealed a coordination self-adaptation mechanism for Li ions to occupy under-coordination sites and inherit the wide bottleneck from NZSP precursor, which plays the key role in enhancing ionic conductivity, in theory.
This cationic exchange was experimentally and theoretically proved to make Li-ion occupied in tetrahedral sites in order to maintain structural stability and the migration bottleneck sizes of Li ions are significantly increased due to inherited structure from Na-ion solid electrolyte.
As a result, the synthesized LZSP was demonstrated to not only have the highest Li-ion ionic conductivity at room temperature in oxide solid electrolytes, but also present considerably stability for Li metal and air.
The proposed method of skeleton-retained cationic exchange in this study is different from commonly adopted molten salt ion-exchange method, by which phase transformation may take place and result in low ionic conductivity, and could be commonly used to produce more solid electrolyte, even extensive inorganic materials, by controlling suitable reaction conditions.
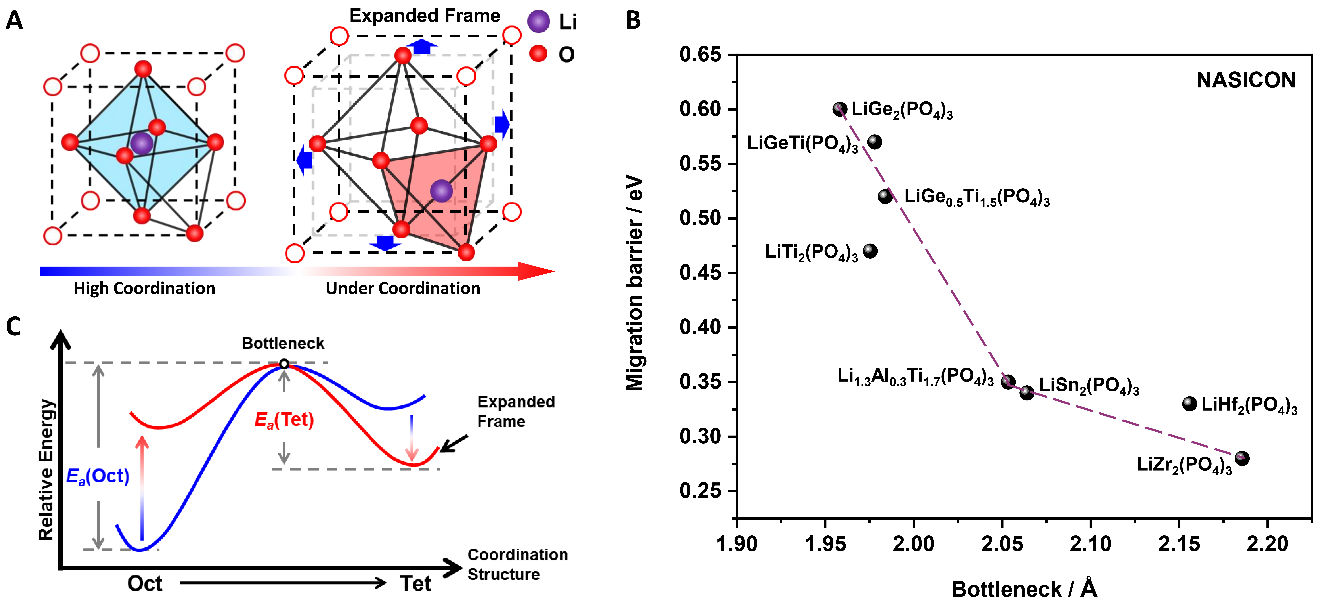
Fig. 1. Relationships among coordination modulation, bottleneck and migration energy barrier. (A) Structural schematic of coordination engineering for the transition between LiO6 hexahedrons and LiO4 tetrahedrons. (B) Migration barrier of Li+ ions from LiO6 hexahedrons to LiO4 adjacent tetrahedrons. (C) Relations between bottleneck and the migration energy barrier of lithium-ion in NASICON-type lithium solid electrolytes. (Image by LIU Jianjun)
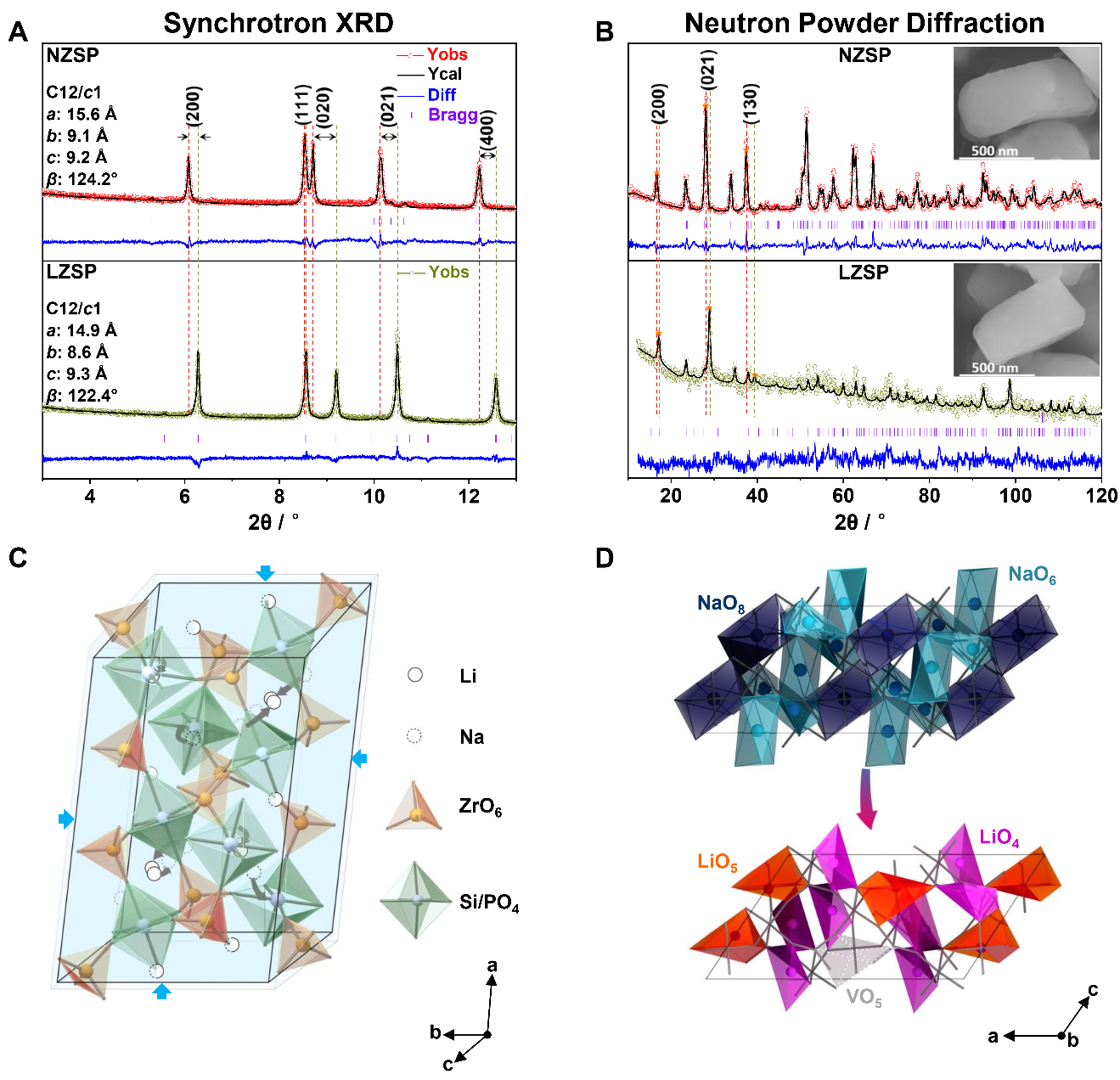
Fig. 2. Crystal structures of NZSP and LZSP. (A) Refined powder synchrotron XRD and (B) NPD patterns of NZSP and LZSP powders with Rietveld method. The SEM images of NZSP and LZSP are inserted in B. All these patterns are recorded at room temperature. (C) The retained polyhedral skeletons containing the evolution path of Na+ ions to Li+ ions. (D) The evolution of Na+ ions to Li+ ions polyhedrons within unchanged skeleton. (Image by LIU Jianjun)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

