
Regulatory T (Treg) cells play a critical role in the maintenance of immune homeostasis but are also involved in the generation of suppressive tumor microenvironments. Similar to effector T cells, Tregs undergo an activation process to gain increased inhibitory activity and eventually differentiate into effector Tregs, which can produce various soluble inhibitory cytokines and surface molecules to suppress the immune response.
Aside from the well known inhibitory cytokines such as TGF-β and IL-10, the new immunosuppressive cytokine IL-35 has been implicated in Treg cell function through a combination of bystander suppression and infectious tolerance. However, the study of IL-35 in relation to immune regulation is limited to RT-PCR and ELISA assays due to the lack of a suitable antibody to detect IL-35-expressing cells.
In a study published online in Cell Reports, Dr. ZHOU Xuyu and his colleagues from Institute of Microbiology of Chinese Academy of Sciences generated a novel IL-35 reporter mouse (Ebi3-Dre-Thy1.1) in which the surface expression of Thy1.1 reflects intracellular IL-35 secretion. Using this transgenic mouse, they could study the function of IL-35-expressing cells in vivo by injection of the anti-Thy1.1 antibody.
Researchers found that the IL-35-expressing cells are mainly derived from thymic Treg (tTreg) cells. The transcription factor Foxp3 promoted IL-35 secretion in IL-35-Tregs, which was totally independent of the expression of Blimp1.
Subsequent analyses of surface molecules and transcriptional profiles demonstrated that IL-35-Tregs are a distinct Treg lineage from the Blimp1-dependent IL-10-producing subset. IL-35-Tregs expressed high levels of C-C chemokine receptor type 7, tended to stay in the T cell zone, and likely inhibited the early stage of T cell activation.
In contrast, the IL-10-Tregs highly expressed IL-10, granzymes, and a variety of chemokine receptors, tended to migrate to peripheral immune locations, and repressed the immune response. Depletion of both IL-35-Treg and IL-10-Treg cells in mice led to the development of a spontaneous colitis phenotype.
Together, this study elucidated a novel effector subset of Tregs, IL-35-Tregs, and provided evidence of Treg activation status tuning the generation of distinct effector Treg subsets, which work cooperatively to maintain immune tolerance (see Figure). The results may have important implications in both autoimmune pathogenesis and tumor therapy.
This work was granted by the National Natural Science Foundation of China and National key basic research project.
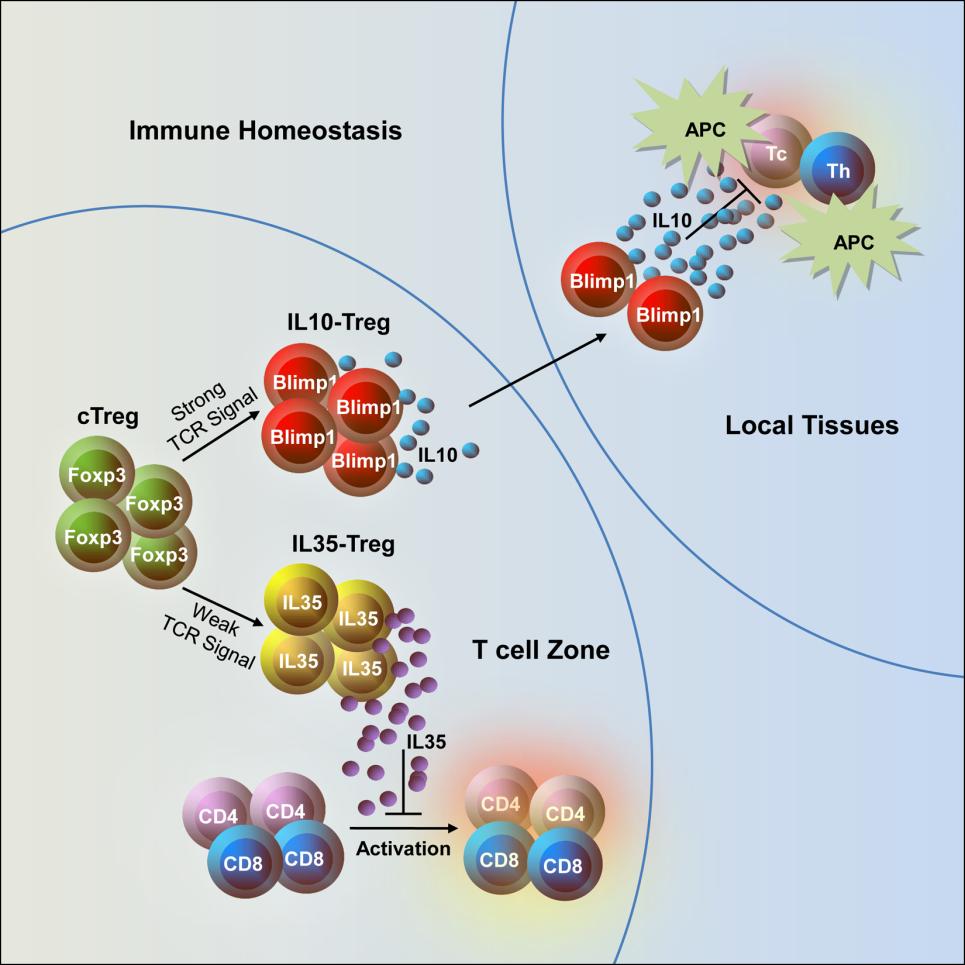
Figure: Distinct effector Treg subsets work cooperatively to maintain immune tolerance (Image by Prof. ZHOU’s lab)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

