Peptide signaling is a key component of cell-to-cell communication in plants. Phytosulfokine (PSK) is a pentapeptide (Tyr-Ile-Tyr-Thr-Gln) with a sulfate group attached to each of the two tyrosine residues and has a ubiquitous role in plant growth and development. It has been found that PSK is perceived by its receptor PSKR, a leucine-rich repeat receptor kinase (LRR-RK). But how PSK is perceived by PSKR and the downstream components are still unknown.
To reveal the PSK activation mechanism, researchers in YANG Weicai’s group from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, collaborated with researchers in CHAI Jijie’s group in Tsinghua University reported the crystal structures of the extracellular LRR domain of PSKR in free, PSK- and co-receptor-bound forms.
Biochemical, genetic and structural evidence revealed that PSK binding on the island domain of PSKR1 enhanced PSKR heterodimerization with the somatic embryogenesis receptor-like kinases (SERKs). PSK was not directly involved in PSKR–SERK interaction but stabilized PSKR island domain for recruitment of a SERK.
This is a new feature from the previous reported peptide-receptor activation mechanism. This result will improve the understanding of plant peptide-receptor perception and activation mechanisms and help to design PSKR-specific small molecules.
This work with WANG Jizong and LI Hongju as the first coauthor has been published on
Nature (doi:10.1038/nature14858). This research was funded by the National Natural Science Foundation of China and the Ministry of Science and Technology of China.
CONTACT:
YANG Weicai, Principal Investigator
Institute of Genetics and Developmetnal Biology, Chinese Academy of Sciences,
Beijing, China.
E-mail:
wcyang@genetics.ac.cn 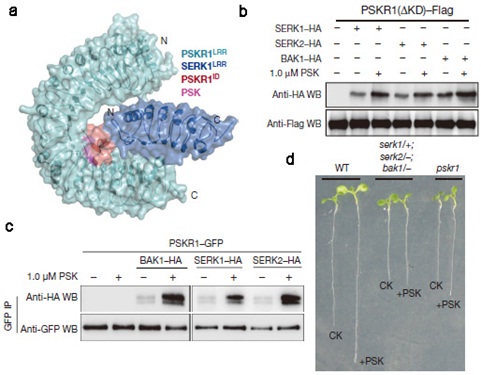
Figure. (a) Overall structure of PSK–PSKR1LRR complex. (b) PSK promotes PSKR1–SERK interaction in Arabidopsis protoplasts. Flag-tagged PSKR1 (∆KD) and HA-tagged SERK1/2/BAK1 were co-expressed in Arabidopsis protoplasts, and their interactions were detected by co-immunoprecipitation. (c) PSK promotes PSKR1–SERK interaction in planta. Crude protein extracts from the treated and untreated plants overexpressing PSKR1–GFP and SERK1/2/BAK1–HA were used for co-immunoprecipitation. (d) The serk1/1; serk2/-; bak1/- triple mutants are less sensitive to PSK in root growth. (Image by IGDB)







