
Adaptor protein (AP) complexes play central roles in intracellular vesicular trafficking by coupling cargo selection to vesicle formation. AP-4, an important member of the AP family, plays a key role in this process. AP-4 dysfunction disrupts the transport of essential cargo proteins, such as ATG9A, leading to their abnormal retention within cells. However, the mechanistic details of how AP-4 is recruited to membranes and how its structural features support this process have remained unclear.
In light of this, a collaborative team led by Profs. FENG Wei and ZHAO Yan from the Institute of Biophysics of the Chinese Academy of Sciences has systematically elucidated the conformational dynamics of the AP-4 core complex and uncovers the molecular mechanisms governing its membrane recruitment and cargo transport by combining cryo-electron microscopy, biochemical analyses, and cellular assays.
Their findings were published in Nature Communications on January 21.
The researchers first reconstituted the soluble AP-4 core complex in vitro and determined its three-dimensional structure using single-particle cryo-EM. The structural data showed that AP-4 is not a rigid, static assembly; instead, it exists in a dynamic equilibrium between "closed" and "open" conformational states.
Further analysis revealed that this structural plasticity primarily stems from a relatively loose interface between the medium subunit μ4 and the core scaffold, providing a structural basis for conformation switching during function.
Subsequent cryo-EM reconstruction of the AP-4/ARF1 complex demonstrated that ARF1 does not activate AP-4 by locking it into a single conformation. Rather, ARF1 appears to modulate membrane recruitment within this dynamic conformational landscape.
Single-molecule FRET measurements independently validated the conformational flexibility of AP-4. Together with structure-guided mutagenesis of key interaction residues, these results confirmed that the AP-4-ARF1 interface is essential for efficient membrane recruitment.
The study also showed that disrupting the intrinsic conformational equilibrium of AP-4 weakens the cooperative action of ARF1 and ATG9A during membrane recruitment, leading to the aberrant intracellular localization of ATG9A.
Based on the integrated structural, biochemical, and cellular evidence, the researchers proposed a working model in which efficient AP-4 membrane recruitment and vesicle formation depend on the synergistic engagement between ARF1 and cargo proteins. Loss of AP-4's conformational dynamics disrupts this synergy and ultimately impedes vesicular transport.
By redefining how AP-4 is recruited to membranes from a conformational regulation perspective, this study provides important molecular insights into AP-4-related neurodevelopmental disorders. It also offers a structural and mechanistic framework for a deeper understanding of vesicular trafficking regulation.
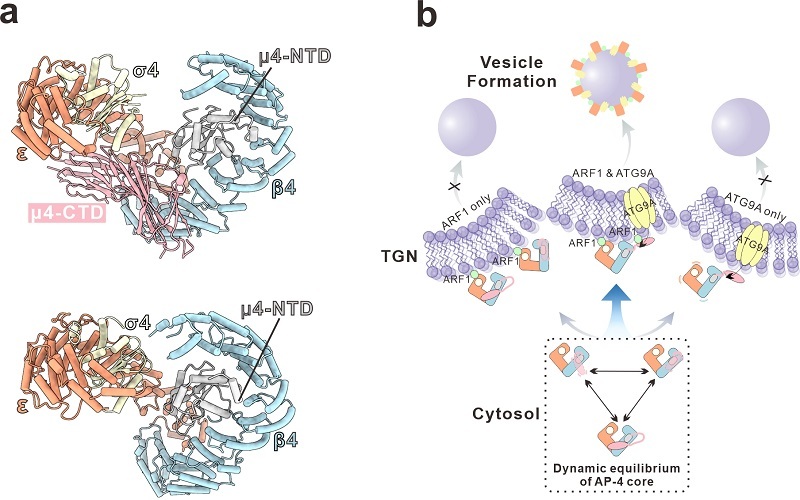
Cryo-EM structure of the AP-4 core complex and mechanistic model of its membrane recruitment (Image by FENG Wei's group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

