
Newsroom
A recent study, conducted by researchers from the Beijing Institute of Genomics at the Chinese Academy of Sciences (CAS), the Institute of Zoology at CAS, and Sichuan University, has produced the first "molecular movie" that illustrates how organs age. The researchers tracked over 12,700 proteins across 13 different tissues from 76 individuals aged 14 to 68 using ultra-sensitive mass spectrometry and artificial intelligence. Their findings were published in the journal Cell on July 25.
The researchers discovered that impaired protein quality control—particularly in blood vessels—lies at the core of systemic aging, with molecules released by aging arteries accelerating decline in distant organs. Long viewed as a patchwork of independent cellular clocks, aging is instead better understood as a single, interconnected network orchestrated by the vascular system, the study shows.
By mid-life, the aorta—the body's largest artery—begins releasing a distinct set of "senoproteins," including Recombinant Growth Arrest Specific Protein 6 (GAS6), which act like systemically delivered aging instructions. When the team injected GAS6 into healthy middle-aged mice, the animals experienced rapid declines in grip strength, balance, and vascular health within weeks.
The researchers identified three key signatures of aging. First, the central dogma of molecular biology frays: the tight link between RNA instructions and their encoded proteins weakens with age, depriving cells of the right tools at the right time. Second, the cellular machinery for producing, folding, and disposing of proteins—including ribosomes, chaperones, and proteasomes—declines across nearly all organs. Third, toxic proteins such as amyloids, immunoglobulins, and complement factors accumulate, weaving an inflammatory network that forms the molecular basis of "inflammaging." A particularly one is Serum Amyloid P (SAP), flagged as the top "universally upregulated pan-tissue aging protein." In lab experiments, SAP alone was enough to push young blood vessel cells into an aged, inflammatory state.
To translate these molecular patterns into practical tools for measuring age, the team developed an AI-driven "proteomic aging clock" for each tissue. These clocks show that the adrenal glands and aortas are the first organs to deviate from their youthful profiles, around age 30—suggesting early hormonal or vascular changes may set the pace for whole-body aging. A dramatic "molecular cascade storm" between ages 45 and 55 marks the shift from piecemeal to systemic aging; during this period, the aorta's protein profile undergoes the most drastic remodeling, and its secreted factors mirror changes in circulating blood, confirming its role as the body's "senohub."
Beyond GAS6, the study identified four additional vascular-derived proteins—glycoprotein non-metastatic melanoma protein B (GPNMB), Cartilage Oligomeric Matrix Protein (COMP), high-temperature requirement serine peptidase 1 (HTRA1), and insulin-like growth factor binding protein 7 (IGFBP7)—that can independently trigger cellular senescence. Injecting GPNMB into young mice recapitulated whole-body aging, reinforcing the principle of "spreading senescence," where a single aging organ transmits aging signals through the bloodstream.
Collectively, this study establishes a "Protein Imbalance–Vascular Hub" model, reframing aging from a collection of failing cells to a communicable, organ-level phenomenon.
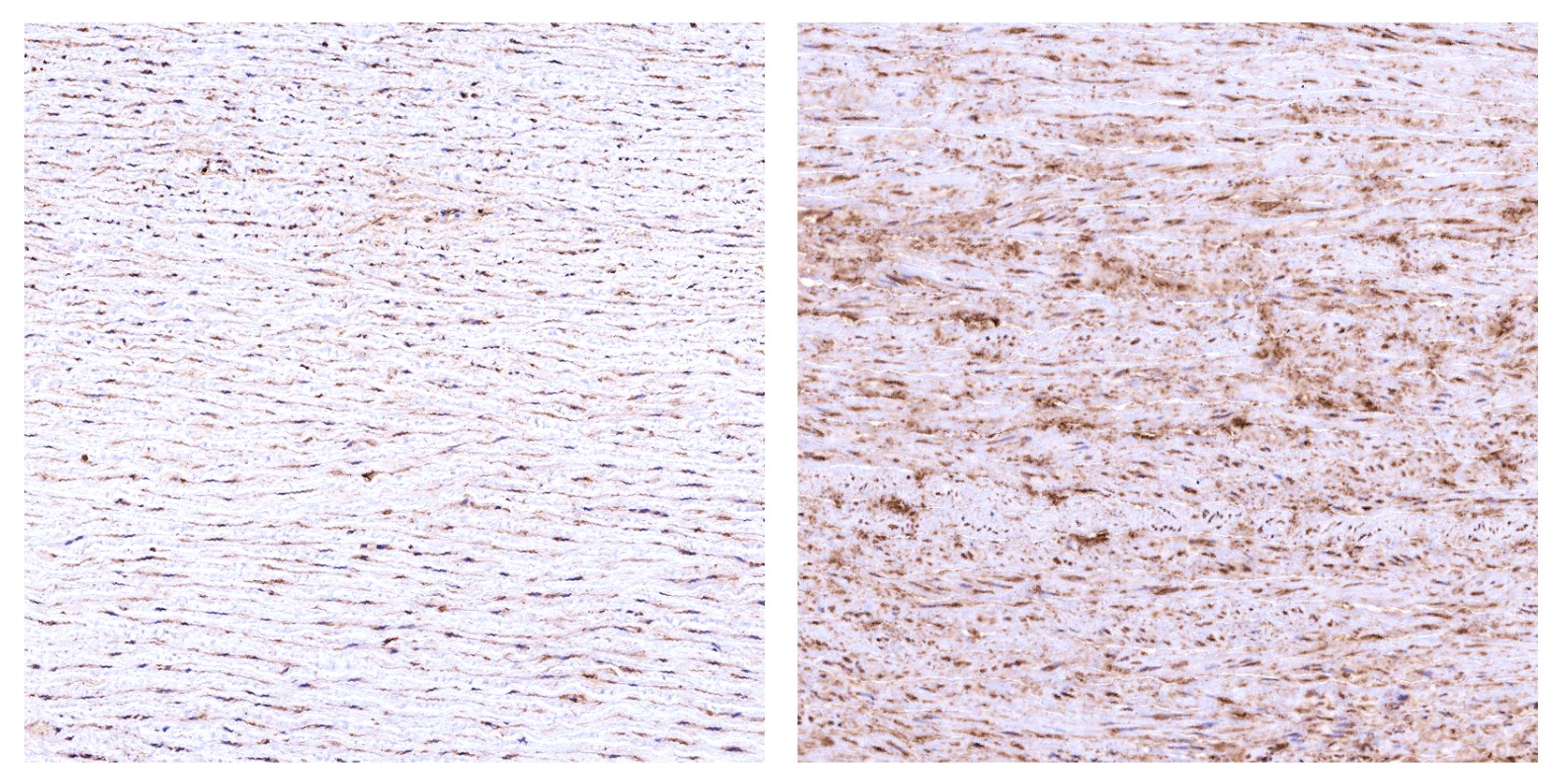
GAS6 rises with age in human aorta: young (left) vs. aged (right) (Image by Profs. LIU Guanghui and ZHANG Weiqi's labs)
