Newsroom
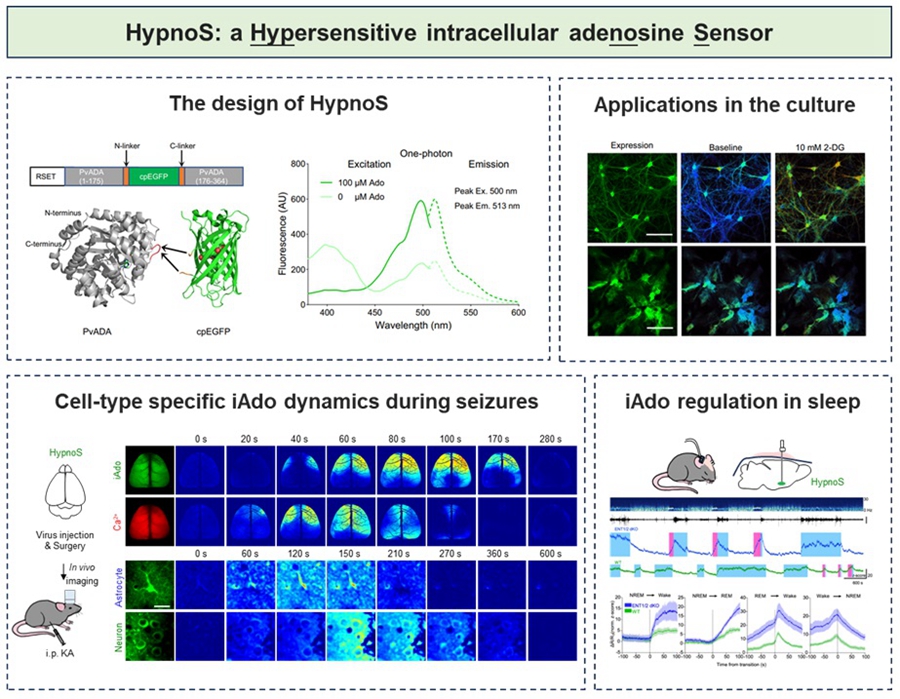
To overcome these challenges, the researchers engineered HypnoS using circularly permuted enhanced green fluorescent protein (cpEGFP) and adenosine deaminase (PvADA). After screening over 3,000 protein variants, they produced a sensor with a maximum fluorescence response amplitude of approximately 900%, sub-second kinetic response, and high substrate selectivity—all without disturbing normal cellular physiology. HypnoS enables visualization of iAdo dynamics at multiple scales, from single cells to whole-brain tissue in live fruit fly and mouse models.
During pathological processes such as epilepsy, adenosine is considered an important endogenous anticonvulsant factor. By combining HypnoS with in vivo wide-field imaging techniques, the researchers mapped the dynamic changes of intracellular adenosine during epileptic seizures in live mouse brains, with the entire cortex as the spatial scale and high-temporal-resolution. The results revealed a potential neuroprotective role of intracellular adenosine in epilepsy. Furthermore, the researchers specifically expressed HypnoS in neurons and astrocytes and employed in vivo two-photon imaging techniques to parse the dynamic changes of intracellular adenosine in different cell types at single-cell resolution.
In physiological processes such as sleep-wake regulation, adenosine is recognized as a key molecule in sleep homeostasis regulation. The researchers combined HypnoS with fiber optics and electroencephalogram and electromyogram recordings, captured the phenomenon of increased intracellular adenosine during wakefulness and rapid eye movement sleep and decreased levels during non-rapid eye movement sleep in the basal forebrain of mice. They found that ENT1/2 primarily mediates adenosine release in neurons and promotes adenosine uptake in astrocytes, revealing the division of labor between the two cell types in sleep homeostasis. This finding provides a molecular basis for intervening in sleep disorders through adenosine-level regulation.
This study was supported by CAS, the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and the "Youth Talent Support Project" of the Beijing Association for Science and Technology.