
Newsroom
Hepatocellular carcinoma (HCC), the third leading cause of cancer-related deaths worldwide, remains one of the most aggressive malignancies. In a recent study published in Nature Communications, researchers led by Profs. YANG Pengyuan and CHEN Runsheng from the Institute of Biophysics of the Chinese Academy of Sciences have uncovered a novel mechanism by which tumor-derived long noncoding RNA (lncRNA) suppresses anti-tumor immunity, providing new insights into the role of extracellular vesicles (EVs) in HCC progression.
The researchers identified HDAC2-AS2 as the most significantly differentially expressed lncRNA following TGF-β stimulation. Although its overexpression or knockdown did not impact tumor cell proliferation, it markedly promoted subcutaneous tumor growth in C57 mice.
Mechanistic studies revealed that tumor-derived HDAC2-AS2 is secreted into the tumor microenvironment via EVs. Higher levels of HDAC2-AS2 were also detected in EVs from the plasma of HCC patients.
Immune function analysis showed that HDAC2-AS2 in EVs was taken up by CD8+ T cells, where it binds to intracellular cyclin-dependent kinase 9 (CDK9), leading to a reduction in intracellular CDK9 protein levels. This, in turn, induces CD8+ T cell exhaustion and apoptosis while suppressing their cytotoxic function.
Multi-omics analysis revealed that CDK9 plays a key regulatory role in CD8+ T cell activation and cytotoxicity. Single-cell RNA sequencing data from HCC patients undergoing immune checkpoint blockade (ICB) therapy showed that CDK9 enhances CD8+ T cell function during ICB treatment.
Additionally, HCC tumors with high HDAC2-AS2 expression were found to benefit more from PD-1 antibody therapy.
This study demonstrates that in HBV-associated HCC, the lncRNA HDAC2-AS2, upregulated by the TGF-β signaling pathway, targets CDK9 in CD8+ T cells via EVs, inhibiting CD8+ T cell function and promoting tumor immune evasion.
These findings provide new potential biomarker and therapeutic targets for HBV- associated HCC. It provides critical insights into the molecular mechanisms of HCC immune evasion and opens new possibilities for precision medicine approaches.
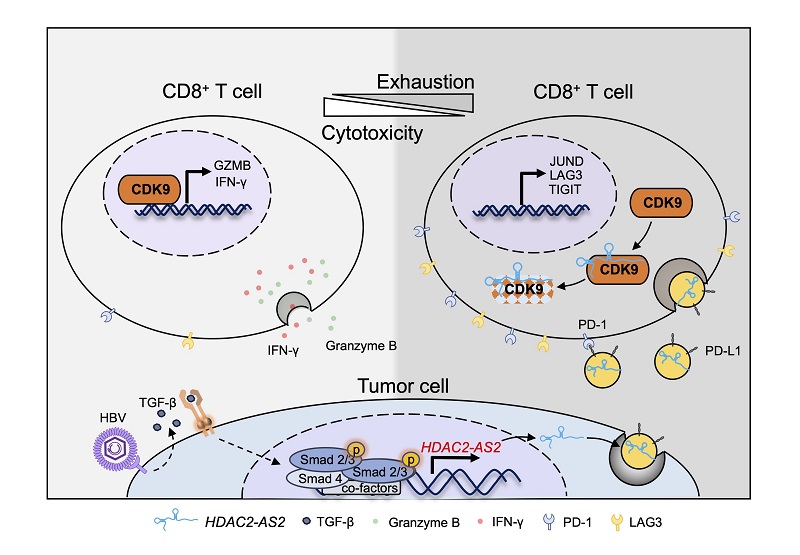
HDAC2-AS2 in tumor-derived extracellular vesicles inhibits CD8+ T cell function by targeting cytosolic CDK9 (Image by YANG Pengyuan's group)
