
Newsroom
A research team led by Prof. ZHAO Yan at the Institute of Biophysics of the Chinese Academy of Sciences has made significant strides in our understanding of the vesicular acetylcholine transporter (VAChT). Utilizing advanced cryo-electron microscopy (cryo-EM) single-particle techniques, the researchers successfully reconstructed high-resolution structures of VAChT in three states: the apo state, the acetylcholine (ACh)-bound state, and the inhibitor vesamicol-bound state.
Published on January 13, 2025, in Nature Structural & Molecular Biology, these findings shed light on the mechanisms behind VAChT’s substrate recognition and proton-coupled transport. The study also provides critical structural insights into how vesamicol interacts with VAChT, offering a foundation for the design of highly efficient and selective radioactive ligands aimed at targeting this transporter.
VAChT is an crucial transporter that facilitates the uptake of acetylcholine into synaptic vesicles for its release. However, the precise molecular mechanisms governing how VAChT selectively transports acetylcholine have remained largely unknown.
The researchers determined the lumen-facing structures of VAChT at resolutions of 3.4 Å in apo state and 3.3 Å in ACh-bound state. Their analysis uncovered a unique hydrogen bond network that distinguishes VAChT from other proteins within the same family.
To further investigate VAChT's transport capabilities, the researchers employed an acetylcholine sensor, GRABACh3.0, to examine both VAChT and its mutant variants. Their findings revealed a coupling relationship between proton binding and substrate transport, offering vital insights into VAChT’s role in the transport, storage, and release of acetylcholine.
Additionally, the study focused on vesamicol, a well-known VAChT inhibitor distinguished by its selectivity and high affinity. By analyzing the high-resolution structure of the vesamicol-VAChT complex, researchers discovered that vesamicol inhibits acetylcholine transport by obstructing VAChT's transition from a lumen-facing conformation to a cytoplasm-facing conformation.
This study enhances our understanding of VAChT substrate recognition and its distinctive proton-coupled substrate transport mechanism. These insights lay an important foundation for the development and optimization of drugs and tracers for neurodegenerative diseases.
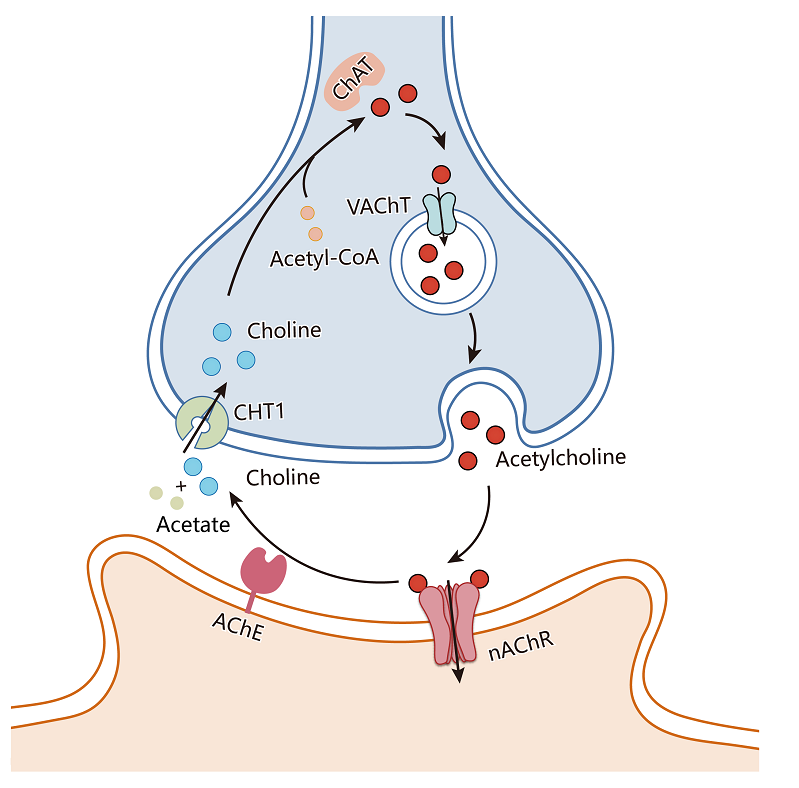
Figure 1. Transmission process of neurotransmitters in cholinergic neurons (Image by ZHAO Yan's group)
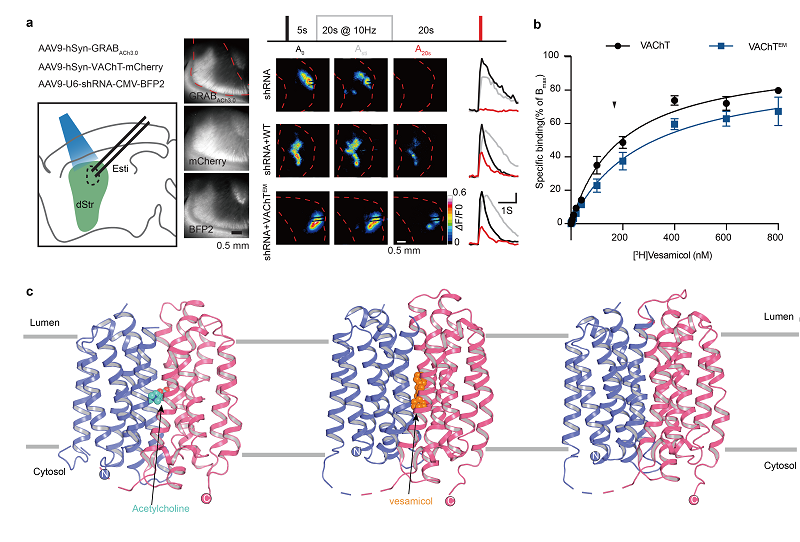
Figure 2. Functional characterization and architecture of human VAChT in apo state, acetylcholine-bound state, and the inhibitor vesamicol-bound state (Image by ZHAO Yan's group)
