
Itaconate is a byproduct of the tricarboxylic acid cycle and an emerging metabolic small molecule that regulates macrophage inflammation. It has drawn high attention for its potent anti-inflammatory effects in recent years.
A Chinese research team has recently traced the discovery of itaconate as an immune modulator and effector molecule, described the development of fluorescent probes for itaconate, and summarized recent research findings on the mechanisms of itaconate transport between cells in a review article published in Cell Insight on Nov. 22.
Itaconate is produced by the mitochondrial enzyme aconitate decarboxylase 1 (ACOD1), encoded by the immune-responsive gene 1 (IRG1), by catalyzing the decarboxylation of the tricarboxylic acid cycle intermediate cis-aconitate. It can also undergo catabolism within mitochondria (Figure 1).
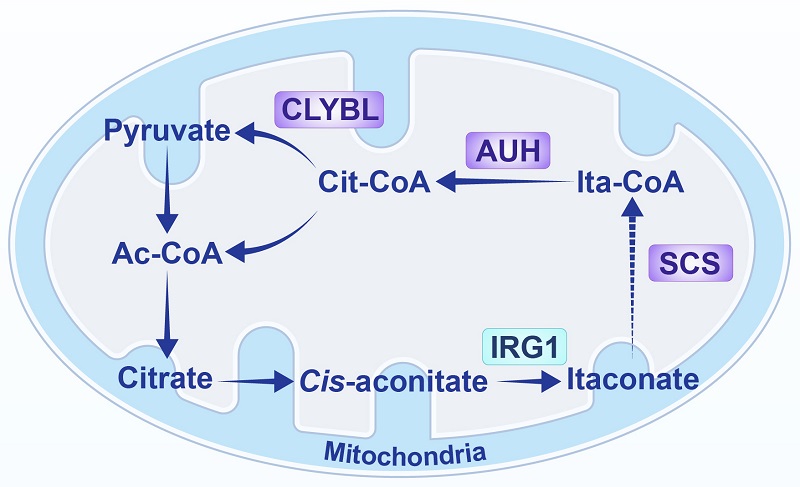
Fig. 1 The metabolic pathway of itaconate in mammalian cell mitochondria (Image by LI Xinjian's group)
Previous studies by by Prof. LI Xinjian's team at the Institute of Biophysics of the Chinese Academy of Sciences have demonstrated that itaconate can activate the transcription factor TFEB through alkylation, inducing lysosome biogenesis and enhancing the antibacterial innate immune capability of macrophages.
Myeloid cells, such as macrophages, can produce itaconate and secrete it extracellularly, suggesting that itaconate generated by myeloid cells might be transported to non-immune cells to exert its functions.
To test this hypothesis, LI and his team developed a genetically encoded fluorescent probe for itaconate, called BioITA. Using BioITA for high-throughput screening of cells expressing a CRISPR gRNA library targeting membrane proteins, they identified ABCG2, an ATP-binding cassette transporter, as the extracellular transporter, and SLC13A3, a solute carrier family protein, as the intracellular transporter of itaconate.
Functional studies revealed that extracellular itaconate release mediated by ABCG2 limits lysosome biogenesis and antibacterial innate immunity in macrophages. Conversely, intracellular uptake of itaconate via SLC13A3 enhances the antibacterial innate immunity of the liver.
These findings elucidated the molecular mechanisms of itaconate's intercellular transport (Figure 2) and suggested that itaconate acts as an immune signaling metabolite.
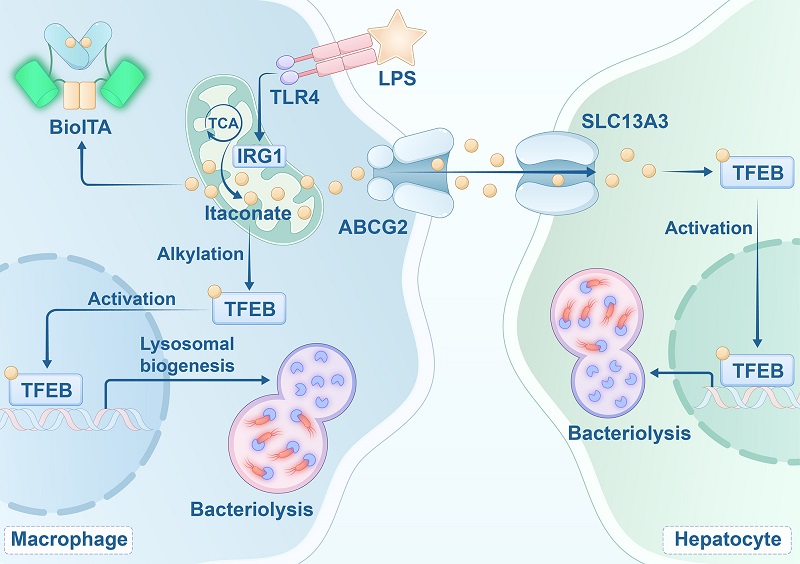
Fig. 2 The intercellular transport mechanism of itaconate (Image by LI Xinjian's group)
The researchers emphasized that the interplay between metabolic changes and immune cell activation is critical for regulating the host's immune response. Beyond serving as a traditional energy source and biosynthetic precursor, metabolites can function as signaling molecules to transmit information between cells.
The discovery of the intercellular transport mechanisms of itaconate highlighted its role as a metabolite with immune messenger properties.
Based on current research progress, the researchers proposed that itaconate is an immune signaling molecule that plays a vital role in host inflammatory responses and immune defense.

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

