
In a study published in PNAS on June 18, a research team led by Eric H. Xu (XU Huaqiang) and ZHAO Lihua from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, employing single-particle cryo-electron microscopy (cryo-EM) techniques, unveiled three-dimensional structures of SSTR5 in complex with the natural neuropeptide agonist cortistatin-17 (CST17) and clinically approved drug octreotide, thus shedding light on molecular mechanisms underlying SSTR5 activation by these ligands.
Researchers meticulously determined cryo-EM structures of SSTR5 bound to CST17 and octreotide at resolutions of 2.7 angstroms and 2.9 angstroms, respectively. These structures revealed that the binding of these agonists triggers a rearrangement of a “hydrophobic lock” formed by the transmembrane helices TM3 and TM6, leading to an outward movement of TM6. This conformational change facilitates the coupling and activation of G protein, initiating downstream signaling cascades.
Remarkably, structural and functional analyses unveiled distinct recognition modes of extracellular loops ECL2 and ECL3 for CST17 and octreotide, providing insights into their agonist selectivity and specificity.
The significance of this study lies in its elucidation of the activation mechanisms of SSTR5 and its selective recognition of neuropeptide and drug agonists. The study paves the way for structure-based design of novel, highly selective SSTR5 modulators with reduced off-target effects, and offers promising therapeutic opportunities for treating a wide range of conditions, including acromegaly, pituitary adenomas, neuroendocrine tumors, and hormonal imbalances.
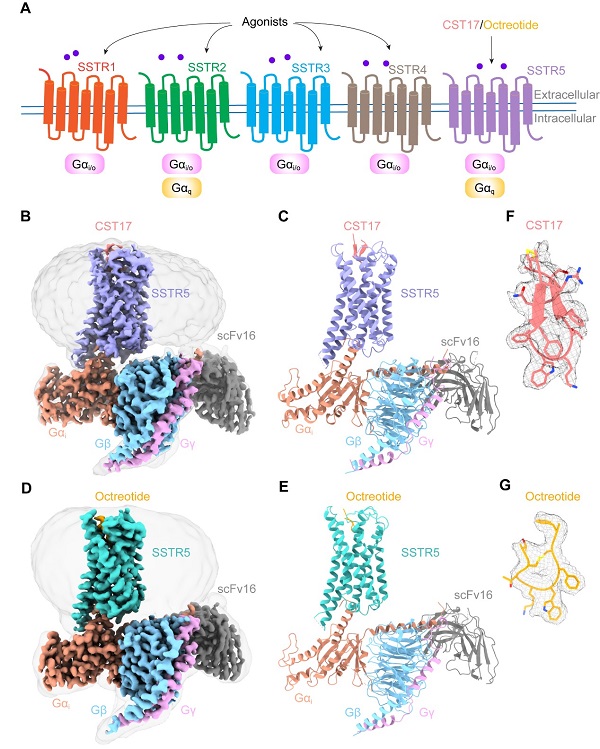
Cryo-EM structures of the SSTR5-Gi complexes bound to the neuropeptide agonist CST17 and the drug octreotide, respectively. (Image by LI Jingru)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

