
Vesicular monoamine transporter 2 (VMAT2) is the only transporter protein in the central nervous system that mediates the storage of monoamine neurotransmitters. It plays a critical role in mediating nerve impulse transmission and neuroprotection. Currently, the mechanisms by which VMAT2 recognizes and transports several structurally different monoamine neurotransmitters and the Parkinson's disease inducer MPP+ are not clear, and the molecular mechanism of proton-coupled substrate transport also needs to be further explored.
In a study published in Cell Research on May 22, a research team led by Prof. ZHAO Yan from the Institute of Biophysics of the Chinese Academy of Sciences (CAS), in collaboration with Prof. JIANG Daohua from the Institute of Physics of CAS, has reported the apo structure of human vesicular monoamine transporter 2 (hVMAT2) at low pH, the complex structures of vesicles facing dopamine, norepinephrine, histamine, and the neurotoxin MPP+ binding, and the complex structure of norepinephrine binding in the cytosol-facing state.
These structures reveal the structural basis of VMAT2 substrate recognition and further refine the molecular mechanism of proton-coupled substrate transport.
Although norepinephrine, dopamine, serotonin, and MPP+ have different molecular structures, the complex structures of VMAT2 binding to these substrates show that they all bind to similar positions on the transporter protein. However, subtle differences in certain functional groups of these substrates lead to significant and critical differences in their interactions and binding modes with the transporter protein. These differences illustrate how VMAT2 efficiently recognizes different substrate molecules.
The researchers reported the structures of norepinephrine binding to VMAT2 in different conformations, including the cytosol-facing and vesicle-facing conformations. Despite significant conformational changes in VMAT2, the substrate binding pocket for norepinephrine remains relatively stable during this process, with no significant changes in interactions with surrounding residues. This clever conformational transition is crucial for the function of VMAT2.
By analyzing the structures of VMAT2 at different pH conditions, it was confirmed that D33 may be another key protonation site.
By thoroughly analyzing the mechanisms by which VMAT2 recognizes different structurally diverse monoamine neurotransmitters and neurotoxins, this work proposed a molecular model for VMAT2 conformational changes and further refined the specific mechanism of proton-coupled substrate transport.
These findings provide valuable insights for a comprehensive understanding of the VMAT2 transport mode, enrich the knowledge system of the major facilitator superfamily substrate transport, and lay an important foundation for drug development and optimization.
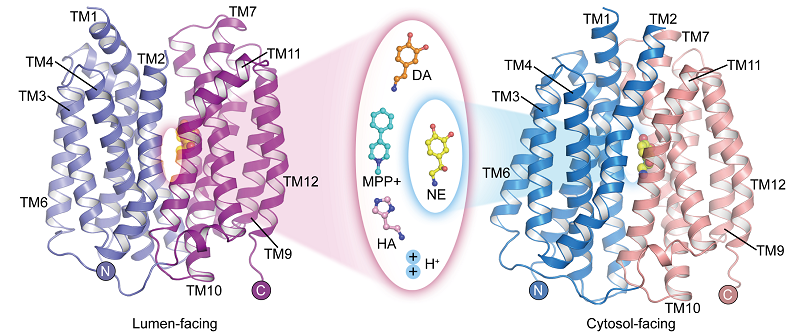
The structure of VMAT2 binding neurotransmitters and neurotoxins. (Image by ZHAO Yan's group)
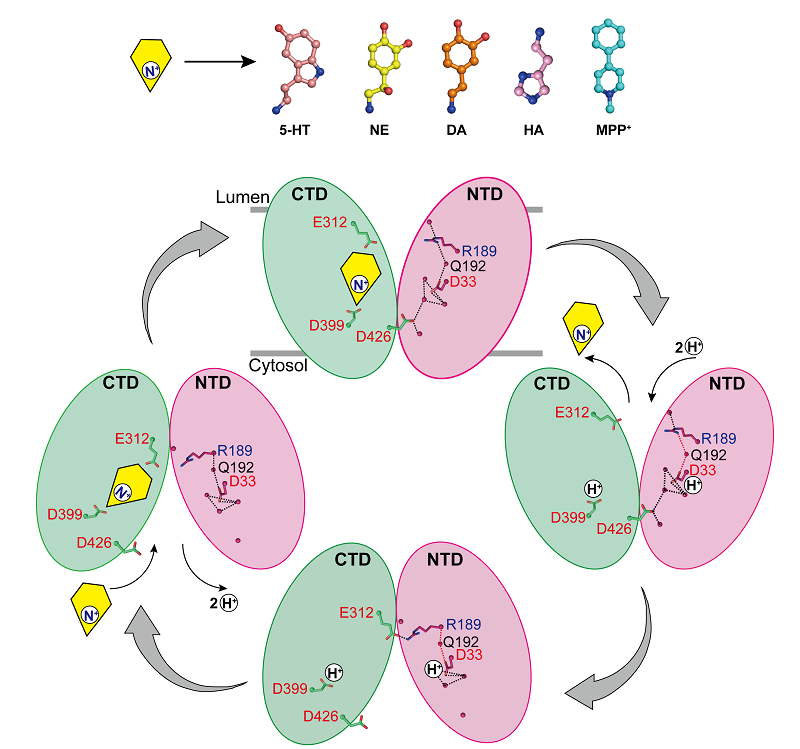
VMAT2 transport monoamine mechanism model. (Image by ZHAO Yan's group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

