
Nicotinamide adenine dinucleotide phosphate oxidase 4 (NADPH oxidase 4, NOX4) is an important member of the NADPH oxidase family that is primarily responsible for the production of H2O2. The regulation of NOX4 activity is predominantly through protein expression. However, the precise mechanisms by which highly secreting cells maintain NOX4 expression and activity while balancing H2O2 levels within the appropriate physiological range remain unclear.
On March 15, a research team led by Prof. XU Pingyong from the Institute of Biophysics of the Chinese Academy of Sciences published a study in Redox Biology, introducing the first negative regulator of NOX4 translation, the pivotal factor EI24. They uncovered the molecular mechanism by which EI24 precisely regulates H2O2 production by controlling NOX4 translation, and its implications for maintaining the redox equilibrium of pancreatic beta cells and insulin synthesis.
The researchers discovered that the endoplasmic reticulum-resident protein EI24 responds to fluctuations in H2O2 levels. Targeted deletion of the Ei24 gene in pancreatic beta cells significantly increased NOX4 protein expression and endoplasmic reticulum H2O2 levels.
Using dual fluorescent reporter systems and immunoprecipitation assays, the researchers showed how EI24 binds to the RNA-binding protein RTRAF and anchors it to the 3'UTR region of the Nox4 mRNA. This interaction inhibits the translation process, effectively controlling the excessive generation of H2O2.
Deletion of EI24 caused RTRAF to translocate to the nucleus, releasing the NOX4 translation inhibition and subsequently affecting the translation of the downstream transcription factor MAFA. As a result, Ei24 knockout reduced the binding capacity of MAFA to the Ins2 promoter, which impaired insulin production and perturbed blood glucose levels in mice.
This study reveals a novel co-translational regulatory system and elucidates how endoplasmic reticulum proteins precisely control the co-translation of membrane-located proteins by modulating the localization of RNA-binding proteins. This regulatory process is of remarkable physiological importance and plays a critical role in maintaining the redox balance and vital functions of secretory cells.
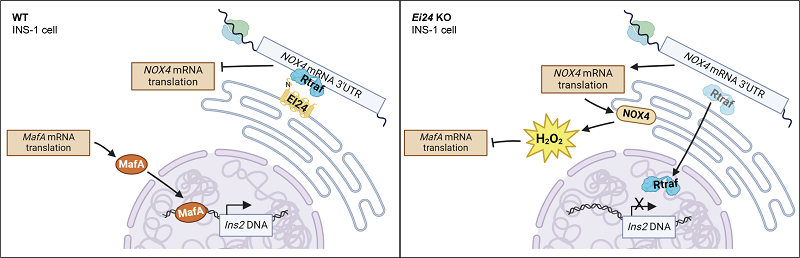
EI24 interacts with the RNA-binding protein RTRAF and Nox4 mRNA 3′-UTR to inhibit the translation of Nox4 in INS-1 cells. (Image by XU Pingyong's group)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

