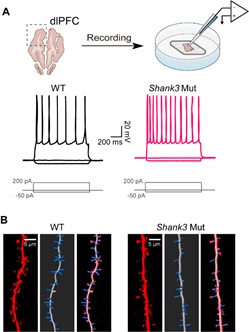
Researchers led by Prof. ZHANG Yongqing from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences have established for the first time a brain slice electrophysiology system in an autism-associated canine model and revealed neuronal structural and functional abnormalities in Shank3 mutant dogs.
SHANK3 mutations are highly correlated with autism spectrum disorders (ASD). In rodents, the absence of Shank3 leads to abnormal behaviors associated with ASD, including social interaction deficits and repetitive stereotyped behaviors. Synaptic dysfunction and dendritic spine reduction in specific brain regions due to Shank3 deficiency are thought to be the neural basis leading to behavioral impairments. Given the significant differences in brain structure and social behavior between rodents and humans, such as human interactions involving complex social cognition and understanding while rodents rely primarily on olfaction for communication, there is a challenge in translating findings from rodent models to human patients.
Dogs, which co-evolved with humans over a long history and possess complex interspecies emotional and social processing capabilities, serve as an effective animal model for studying human social behavior and mental disorders. Therefore, detecting changes in neuronal activity and synaptic function in Shank3 mutant dogs is critical to understanding the causal relationship between Shank3 mutations and abnormal social behaviors.
The researchers used Shank3 mutant dogs as a model animal to study the electrophysiological function and morphological characteristics of neurons in the prefrontal cortex, a brain region highly involved in social cognitive behaviors. The results showed that pyramidal neurons in the prefrontal cortex of mutant dogs exhibited increased excitability and decreased excitatory synaptic transmission compared to wild-type dogs.
In addition, reduced dendritic complexity, a lower dendritic spine density, and smaller postsynaptic density structures were also found in Shank3 mutant dogs. These phenotypes suggest that heterozygous Shank3 mutations are sufficient to induce morphological and functional abnormalities in cortical pyramidal neurons, which may contribute to abnormal social behaviors in mutant dogs.
This study demonstrates the feasibility of using canine brain slices to explore neural circuits and mechanisms of ASD.
Increased neuronal excitability and reduced dendritic spine density in Shank3 mutant dogs (Image by (IGDB)