
Viable bacteria in the blood, i.e., bacteremia, can lead to bloodstream infection (BSI) and sepsis, a syndromic, often fatal, inflammatory response.
Rapid and accurate antimicrobial prescriptions are critical to decreasing mortality in BSI patients. However, traditional antimicrobial susceptibility testing (AST) for BSI is time-consuming and tedious, leading clinicians to rely primarily on their experience when prescribing treatment.
Responding to the need for faster diagnostic tools, researchers from Shandong University, the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS), and the Affiliated Hospital of Qingdao University have developed an integrated microfluidic chip (BSI-AST chip) for rapid AST from positive blood cultures (PBCs). Using the chip, the process from bacteria extraction to getting AST results takes less than 3.5 hours, thus promising to be a powerful new tool in managing bloodstream infections.
The study was published in Analytical Chemistry on Sept. 14.
"Traditional AST methods currently require at least two days to yield results following a positive blood culture. The delay in diagnosis compels the administration of empirical antibiotics, risking the aggravation of the patient's condition and fostering the emergence of antibiotic resistance," said Prof. MA Bo from the Single-Cell Center at QIBEBT, co-author of the study. "Therefore, there is an urgent need for new technologies that can provide accurate and timely diagnostics and drug susceptibility testing."
In this study, the researchers designed a BSI-AST chip capable of extracting bacteria directly from PBCs within 10 minutes—providing rapid AST results requires an additional three hours.
In a proof-of-concept study, the BSI-AST chip demonstrated its effectiveness by conducting direct AST on artificial PBCs containing E. coli, testing against 18 antibiotics, with results in less than 3.5 hours.
Moreover, the integrated chip was applied to the diagnosis of clinical PBCs, showing a categorical agreement of 93.3% with standard clinical methods. The reliable and rapid AST results of the chip highlight its great potential in clinical diagnosis.
"In previous studies, microfluidic devices were mainly designed for purification and concentration of viable microorganisms derived from subculture or urine samples with simple composition," said ZHU Meijia, a doctoral student from Shandong University and first author of the study. "The practical utilization of these devices faced significant challenges due to the absence of on-chip complex sample preparation processes."
XU Teng, assistant research fellow and contributing author from the Single-Cell Center at QIBEBT, said that the BSI-AST chip was a "significant advancement" since it could work directly from PBCs without the need for a subculture.
The researchers achieved rapid extraction and enrichment of bacteria from PBCs by introducing a separator gel to the microfluidic chip for the first time. Centrifugal microfluidic enrichment technology also was central to the process. Furthermore, the chip's multiplexing analysis capability through antibiotic drying and array parallelization support clinicians in optimizing antibiotic therapy for BSI patients.
The BSI-AST chip also provides a rapid and convenient solution for sample pretreatment when combined with Clinical Antimicrobial Susceptibility Test Ramanometry (CAST-R), an instrument that the team invented, according to Prof. XU Jian, the head of the Single-Cell Center at QIBEBT.
"Rapid AST in blood culture is significant for patients with clinical sepsis and has the potential to save lives," said Prof. CHENG Yongqiang of Shandong University, the study's corresponding author. Prof. CHENG also noted the role of such technology in "combating the serious threat of microbial resistance to humanity."
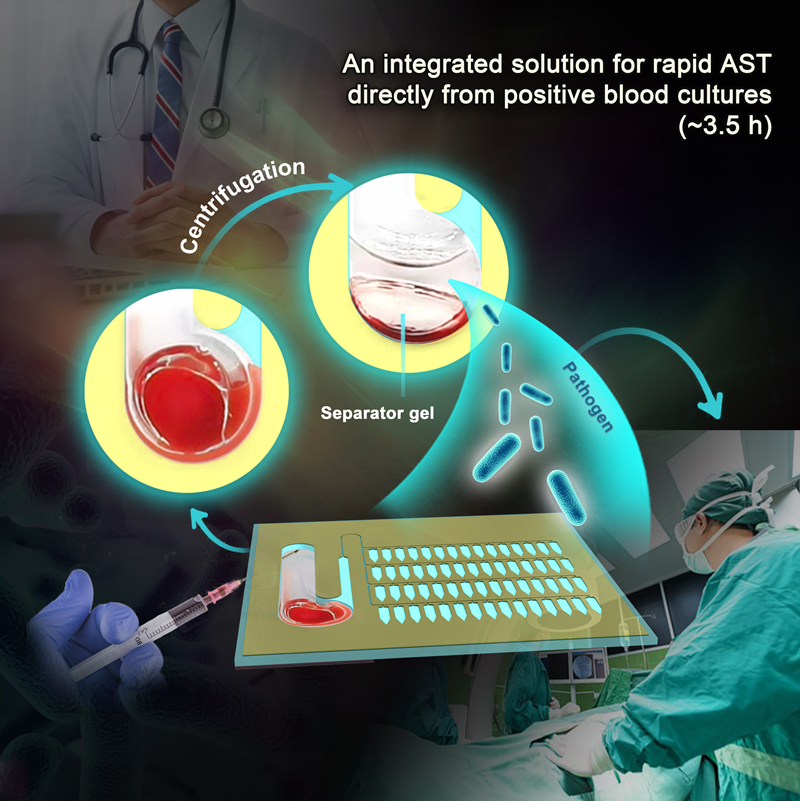
On-chip pretreatment and rapid AST based directly on positive blood cultures (Image by LIU Yang)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

