
G protein-coupled receptors (GPCRs) are essential for cell signal transduction and comprise the largest drug target protein family. Upon agonist stimulation, these receptors activate multiple downstream transducers, including G proteins and arrestins, leading to distinct physiological functions. Arrestins play critical roles in modulating GPCR functions by terminating G protein signaling and promoting internalization.
Class B GPCRs are involved in many severe diseases such as diabetes, obesity and osteoporosis. These receptors are of great interest as targets for developing biased drugs that selectively trigger G protein-dependent pathways or arrestin recruitment, aiming for better efficacy and reduced side effects. However, only a few arrestin-bound GPCR structures have been determined and all belong to the class A GPCR family. Lack of molecular details of arrestin engagement with class B GPCRs limits the understanding of arrestin-mediated modulation of these receptors and hampers drug discovery.
In a study published in Nature, a research group led by Profs. WU Beili and ZHAO Qiang at the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences (CAS) determined the cryo-electron microscopy (cryo-EM) structures of the class B glucagon receptor (GCGR) bound to β-arrestin 1 (βarr1) in glucagon-bound and ligand-free states. These structures provide for the first time a detailed picture of interaction between a class B GPCR and an arrestin and unexpectedly reveal many unique features never before observed.
The GCGR–βarr1 structures display a “tail” conformation of the complex, with the receptor interacting with βarr1 mainly through helix VIII in its C-terminal tail. This is in stark contrast to the previously determined GPCR–arrestin structures in which the arrestin adopts a “core” conformation by binding to both the receptor transmembrane core and the C terminus. The tail-binding pose of βarr1 was further defined by the close proximity between the C-edge loops of βarr1 and the transmembrane helical bundle in GCGR. In addition, a phosphoinositide derivative bridges βarr1 with helix VIII of the receptor to further stabilize the tail engagement.
It was previously suggested that the tail and core conformations of arrestins govern distinct processes of receptor signaling and cellular trafficking, thereby creating different cellular responses. For the first time, however, these GCGR–βarr1 structures offer molecular details of an arrestin coupling to a GPCR in a tail conformation, thus greatly expanding knowledge of the mechanism underlying arrestin-mediated regulation of GPCR signal transduction.
Another striking difference in GCGR-βarr1 structures compared to previously known arrestin-bound GPCR structures is that the receptor exhibits an inactive conformation even in the presence of the endogenous agonist glucagon. The agonist is either absent or loosely attached to the receptor by binding to a shallower binding site than in active GCGR structures.
These structural features are likely due to the tail-binding mode of the arrestin, which lacks any contact with the receptor core and does not require a receptor to retain its active conformation. These findings offer a new opportunity for developing novel biased ligands that preferentially recognize different conformations of GCGR with pathway selectivity.
To further investigate the roles of tail engagement for the arrestin, the scientists performed extensive functional studies using techniques of mutagenesis and bioluminescence resonance energy transfer (BRET). By measuring arrestin recruitment at the plasma membrane and endocytosis of wild-type GCGR and its mutants in the binding interface between the receptor and βarr1, it was confirmed that the tail conformation of the GCGR-βarr complex plays an important role in governing the molecular trafficking of the receptor.
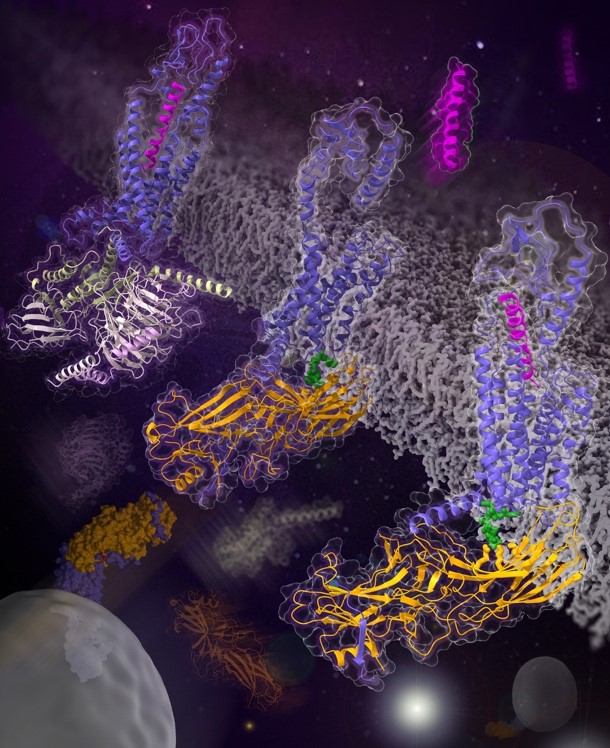
Structures of the human glucagon receptor GCGR involved in glucose homeostasis (Image by WU Beili's laboratory at SIMM)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

