
MI causes irreversible loss of cardiomyocytes and destructs non-myocardium, including the epicardium. In addition, acute MI induces inflammatory cascades via the recruitment of various inflammatory and immune cells, leading to multifaceted processes of myocardial injury and healing. Thus, it is important to identify the reparative strategies by modulation of immune responses via stimulating the endogenous healing process.
Emerging evidence suggests the essential roles of epicardium in regulating coronary artery development via epithelial-mesenchymal transition and the cardiomyocyte proliferation during heart development. The implantation of epicardium-derived cells isolated from human adult atrial tissues preserves left ventricular function and attenuates remodeling in infarcted mouse hearts, while the mechanism is largely unknown. The hEPs are demonstrated to increase maturation of hPSC-derived cardiomyocytes (hCMs) and cardiac graft size as supportive cells when co-transplanted with hCMs. However, it remains unclear whether implantation of hEPs has therapeutic effects on infarcted hearts.
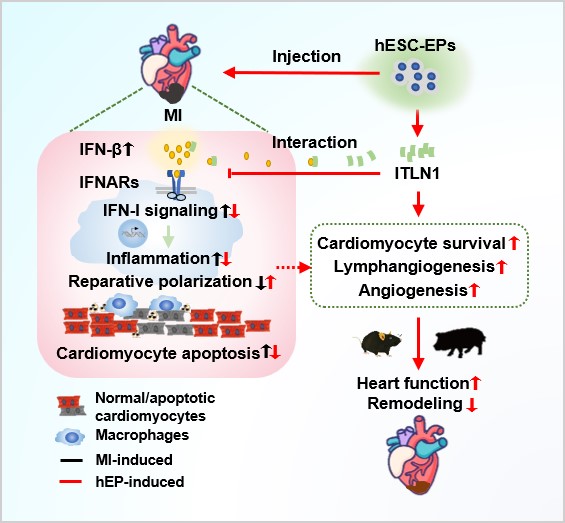
Type I interferons (IFN-I) are cytokines that have antiviral, antiproliferative, and immunomodulatory activities. IFN-I responses are upregulated at the early stage of MI and inhibition of IFN-I responses reduce infarct size. Whether the IFN-I ligands can be directly regulated by paracrine factors and whether hEPs participate in this process remains unknown.
Using the mouse and swine models, the researchers combined with integrated approaches and revealed that intramyocardial injection of hEPs at the acute phase of MI ameliorates functional worsening and scar formation in mouse hearts, concomitantly with enhanced cardiomyocyte survival, angiogenesis, and lymphangiogenesis.
Mechanistically, hEPs suppress MI-induced infiltration and cytokine-release of inflammatory cells and promote reparative macrophage polarization. These effects are blocked by an IFN-I receptor agonist RO8191. Moreover, intelectin 1 (ITLN1), abundantly secreted by hEPs, interacts with IFN-β and mimics the effects of hEP-conditioned medium in the suppression of IFN-β-stimulated responses in macrophages and promotion of reparative macrophage polarization, whereas ITLN1 downregulation in hEPs cancels the beneficial effects of hEPs in anti-inflammation, IFN-I response inhibition, and cardiac repair.
Furthermore, similar beneficial effects of hEPs are observed in a clinically relevant porcine model of reperfused MI, with no increases in the risk of hepatic, renal, and cardiac toxicity.
Collectively, the study revealed hEPs as an inflammatory modulator in promoting infarct healing via a paracrine mechanism and provides a new therapeutic approach for infarcted hearts.
This study entitled “hESC-Derived Epicardial Cells Promote Repair of Infarcted Hearts in Mouse and Swine” was published online in Advanced Science on July 28, 2023. Prof. YANG Huangtian from SINH of CAS and Prof. GAO Ling from Shanghai East Hospital of Tongji University are the corresponding authors.
This work was funded by grants from National Key R&D Program of China, the Strategic Priority Research Program of the CAS, National Natural Science Foundation of China, and the Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai. Technical assistance was provided by the Co-Facilities Center of SINH, CAS and DAI Gonghua from the Department of Radiology, Shanghai East Hospital, Tongji University.

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

