The central node of the cellular metabolic network is the mitochondrion. Mitochondrial stress is closely linked to aging and a number of illnesses, including cancer and neurodegeneration. For the purpose of preserving mitochondrial homeostasis, cells autonomously elicit particular stress responses to deal with mitochondrial stress.
It's interesting to note that tissues or cells not directly experiencing mitochondrial stress can nevertheless be subjected to non-autonomous induction of mitochondrial stress responses. Mitokines, which are secreted chemicals, mediate such non-autonomous mitochondrial stress responses. There is a rise in mitokine research because of their great translational potential in improving human metabolic health.
In a review article published on
Life Metabolism, Dr. Andrew Dillin's group from the University of California, Berkeley, together with Dr. TIAN Ye's group from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences (CAS), summarized recent studies on the mitokines-mediated inter-tissue communication of mitochondrial stress signaling and their effects on regulating the metabolic health.
Insights into understanding how different mitokines coordinate inter-tissue mitochondrial stress signaling and organismal metabolism may help to develop strategies to promote metabolic health, according to the researchers.
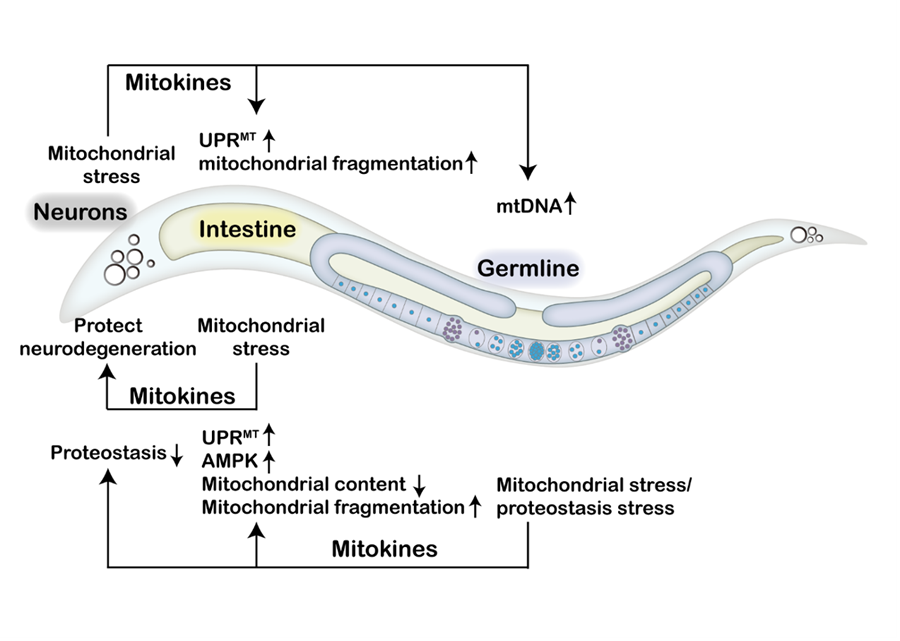
They summarized recent research advances in inter-tissue communication of mitochondrial stress responses in Caenorhabditis elegans. Furthermore, they deeply explored the effects of multiple mitokines on inter-tissue mitochondrial stress signaling and metabolic health in C. elegans and mammals.
They studied examples of mitokines, including fibroblast growth factor 21 (FGF21), growth differentiation factor 15 (GDF15), various mitochondrial-derived peptides (MDP), adrenomedullin 2 (ADM2), and angiopoietin-like 6 (ANGPTL6), as potential biomarkers for metabolic health and how changes in their abundance influence the metabolic status in mammals.
Given that mitochondria may originate from ancient aerobic bacteria, they discussed the possibility that bacterial quorum sensing as an origin of intercellular mitochondrial communication.
Future studies may help develop strategies on utilizing mitokines and intercellular mitochondrial communications to improve metabolic health.
This work was supported by the National Institutes of Health, the Howard Hughes Medical Institute, the National Key R&D Program of China, and the Strategic Priority Research Program of CAS, etc.
Mitokines coordinate inter-tissue communication of mitochondrial stress. (Image by IGDB)