
A joint research team led by Dr. LIU Jingyu and Dr. ZHU Shujia from the Institute of Neuroscience (ION), CAS Center for Excellence in Brain Science and Intelligence Technology (CEBSIT), Chinese Academy of Sciences, has revealed that missense mutations in PiT2-loop7 cause inorganic phosphate (Pi) dyshomeostasis by affecting the phosphorylation-regulated cell-surface localization of PiT2, which finally leads to primary familial brain calcification (PFBC).
The study was published in Neuroscience Bulletin on June 17.
PFBC is an inheritable neurodegenerative disease mainly characterized by symmetrical calcification in the basal ganglia and/or other brain regions. The most common clinical manifestations of this disease include movement abnormality, cognitive impairment, psychiatric symptom, and headache. Until now, no effective treatment has been approved for PFBC patients.
PiT2, encoded by SLC20A2, is capable of transporting extracellular Pi together with Na+ into the cell to maintain Pi homeostasis. Loss-of-function mutations of SLC20A2 were linked to PFBC, and it was proposed that PFBC is caused by Pi dyshomeostasis.
PiT2 mainly consists of two ProDom (PD) domains and a large intracellular loop region (PiT2-loop7). The PD domains are crucial for Pi transport, but the role of PiT2-loop7 remains unclear.
To date, about 147 mutations of SLC20A2 have been identified in PFBC patients. Although most of these mutations are located in the PD domains, some mutations have been identified in PiT2-loop7, the majority of which are nonsense or frameshift mutations that probably cause PFBC due to C-PD1131 deletion. It has been suggested that the PiT2-loop7 domain might not participate in Pi transport, so how did these missense mutations in this domain (p.S283R, p.R382Q, p.T390A, p.H399P, and p.S434W) lead to PFBC?
To explore the detailed molecular mechanisms by which these missense mutations in PiT2-loop7 cause PFBC, LIU's team selected p.T390A and p.S434W in PiT2-loop7 and p.S121C and p.S601W in the PD domains as representatives for investigation.
Radioactive Pi uptake assay and electrophysiological recordings revealed that the missense mutations in PiT2-loop7, similar to those in PD domains, could decrease the Pi transport activity of PiT2. Affinity testing assay demonstrated that the p.T390A and p.S434W mutations in PiT2-loop7 did not affect the substrate-binding ability of PiT2, while the p.S121C and p.S601W mutations in the PD domains affected the Pi- but not the Na+-binding to PiT2.
Combined with bioinformatics analysis, immunoblotting and immunofluorescence, the researchers found that the p.T390A and p.S434W mutations in PiT2-loop7 affected the phosphorylation of PiT2 mediated by AMPK and AKT, respectively, thereby obstructing PiT2 transport to the cell surface.
In PFBC patients, the other missense mutations in PiT2-loop7 (p.S283R, p.R382Q, and p.H399P) could also interfere with PiT2 phosphorylation and membrane localization, reducing the Pi transport activity of PiT2 and leading to PFBC.
This work improves our understanding of PFBC pathogenesis and provides theoretical bases for the development of PFBC therapies.
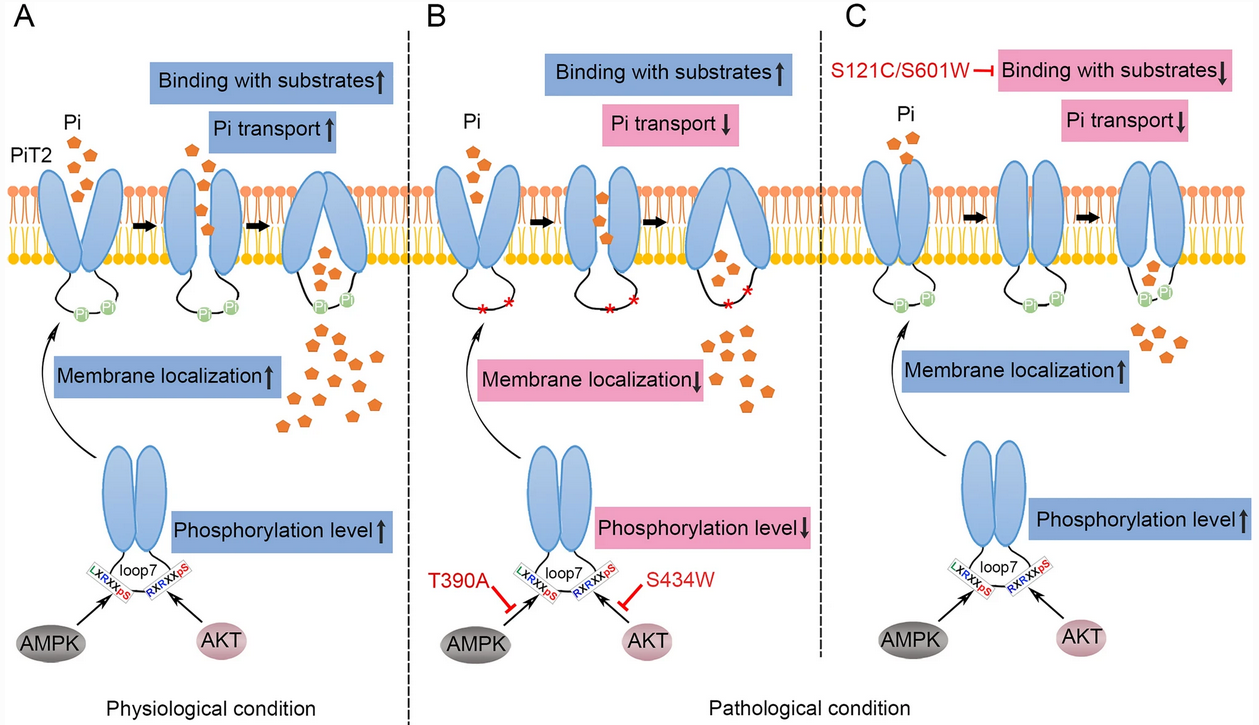
Proposed model of how different mutations in PiT2 cause Pi dyshomeostasis. (Image by CEBSIT)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

