
The oysters, as the representative bivalve mollusc, are widely distributed and support major aquaculture and fishery industries worldwide. However, oysters are still at an early stage of domestication.
Recently, a research team led by Prof. ZHANG Linlin from the Institute of Oceanology of the Chinese Academy of Sciences (IOCAS) proposed a highly effective CRISPR-mediated gene editing technique in the cultured oyster, which provides a powerful tool for genetic engineering breeding to improve productive traits in oysters and other aquaculture species.
Related results were published in Frontiers in Marine Science on May 26.
The researchers conducted CRISPR-mediated knockout by electroporation in Crassostrea gigas angulate with β-tubulin as a marker gene. They detected long fragment deletions in the target gene, and observed mosaic mutations including defective cilia and decreased motility in the first-generation larvae.
Previous strategy majorly generates small indels produced by single sgRNA cleavages, which are usually time-consuming during genotyping. The strategy used in this study co-injected more than two small guide RNAs (sgRNAs) with the goal of generating long fragment deletions, which significantly increases the editing efficiency and simplifies the mutant genotyping workflow by running a normal agarose gel. In this study, a long fragment deletion more than 300 bp was detected in the target gene.
Mosaicism resulting from CRISPR/Cas9 genome editing in animal models is valuable. The team produced mosaic mutagenesis with non-lethal but readily observable phenotypic effects. β-tubulin knockout mediated the mosaic expression patterns of Cgβ-tubulin and mosaic ciliary defects were observed at the positions of peritroch, intestinal cilia and the posterior cilium ring in C. gigas angulate larvae.
"The application of genome editing technologies in oysters calls for establishing efficient CRISPR/Cas9 mutagenesis tool that can generate significant defective phenotypes," said Dr. CHAN Jiulin, first author of the study.
The researchers conducted the CRISPR/Cas9-mediated gene knockout by electroporation and observed the long fragments deletions and mosaic mutations in the first-generation larvae of cultured oyster. The proposed strategy increases mutagenesis efficiency, simplifies the genome editing workflow, and provides a new tool in the molluscs experimental system for gene function studies.
"The application of CRISPR/Cas9-mediated gene editing technology in marine molluscs still faces challenges, either in gene functional or genetic engineering breeding. This study can provide useful reference for a widespread application of gene editing technology in the molluscs in the future," said Prof. ZHANG.
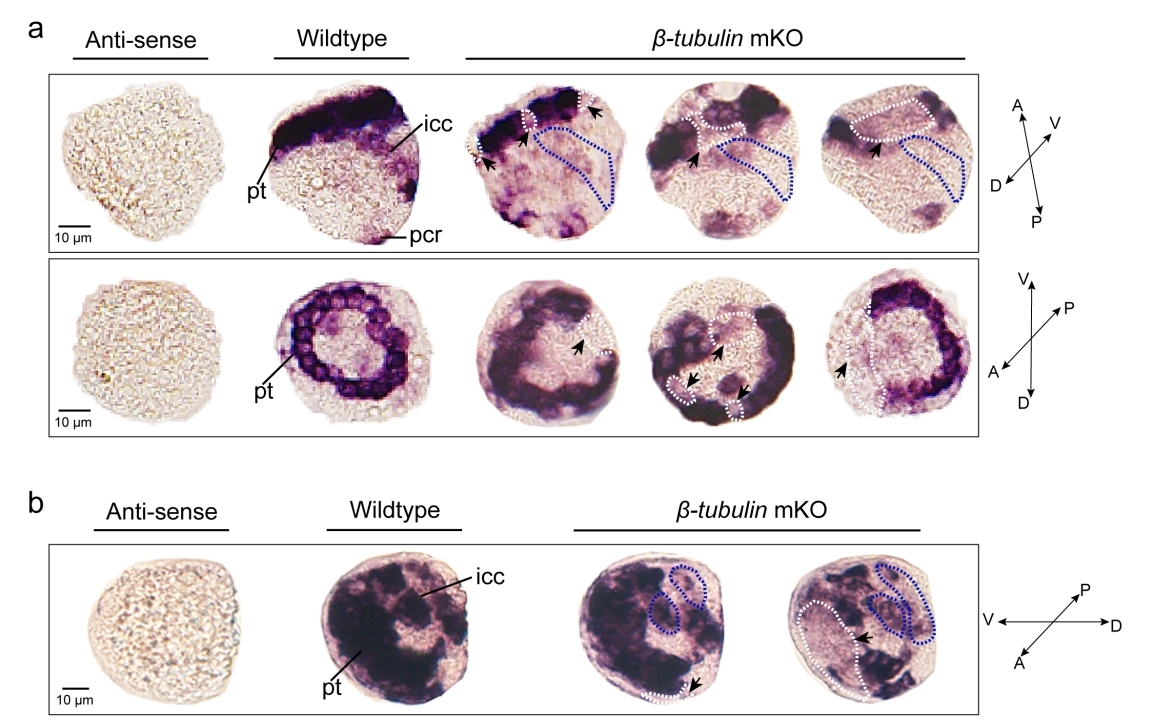
Effects of somatic mutagenesis in oyster larvae (Image by IOCAS)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

