
During pregnancy, the health of the mother and the fetus is dominated by the appropriate allocation of nutrients between the two individuals. Maternal-fetal material exchange predominantly depends on the placenta, which plays critical roles in sensing fetal nutritional demand, modulating maternal supply, and adapting its nutrient transport capacity.
Failures in the regulatory network of placental functions lead to serious clinical complications, such as preeclampsia, recurrent miscarriage, and fetal growth restriction (FGR), etc. FGR is defined as the pregnancy bearing a fetus that does not grow to full potential, largely due to insufficient delivery of maternal nutrition by the placenta. Annually, about 30 million newborns worldwide suffer from FGR, which leads to increased perinatal morbidity and mortality and multiple lifelong health problems.
Lining at the outer surface of the placental villi, the syncytiotrophoblast (STB) is directly bathed in maternal blood, and thus positioned to take charge of maternal-fetal exchanges of gases, nutrients, and waste. STB has been identified as the largest multinucleated epithelial surface in the body, which is formed through cell fusion of the mononucleated cytotrophoblast (CTB). Yet the advantages of such an extensively multinucleated cellular structure in substance exchange remains poorly understood.
A research group led by Prof. WANG Yanling at the Institute of Zoology of the Chinese Academy of Sciences (CAS), in collaboration with Dr. Yoel Sadovsky from University of Pittsburgh and Dr. Bin Cao from Xiamen University, has now revealed a unique strategy of STB to adapt to nutrient deprivation and thus support fetal survival. Their study was published in PNAS on Jan. 6, 2021.
The researchers employed primary human trophoblasts and forskolin-exposed BeWo cells (a trophoblast cell line) as in vitro syncytialization models, they found that macropinocytosis accompanied syncytialization in human trophoblast. Shortage of amino acids markedly promoted trophoblast syncytialization and macropinocytosis through repressing mammalian target of rapamycin (mTOR) signaling.
As macropinocytosis is an efficient route to uptake fluid phase-derived large molecules by at least 10-fold, it empowers placental STB a developmental adaptation to ensure fetal demands when facing nutrient deficiency.
Consistently, the researchers treated pregnant mice with rapamycin, the inhibitor of the mTOR, to generate an animal model of FGR. The mouse placenta exhibited a greater extent of trophoblast syncytialization and enhanced macropinocytosis. Whereas pharmacologically blockage of macropinocytosis in these mice led to greatly worsened fetal growth due to the diminished macropinocytosis-dependent placental transport of large molecule.
The pathological relevance of macropinocytosis in pregnancy complication was revealed in FGR placentas which displayed notable repression in mTOR activity, augment in trophoblast syncytialization and macropinocytosis activity.
Taken together, this study discovered that differentiation of trophoblasts toward syncytium triggers an endocytosis strategy, macropinocytosis, to uptake large extracellular molecules. This unique machinery of nutrient uptake is strikingly boosted via inhibition of mTOR under amino acid shortage conditions, which is essential for fetal survival.
The findings underscore a novel and physiologically important compensatory pathway for placental multinucleated trophoblasts to achieve a prime goal of nutrient delivery in the context of poor blood flow or other limitations in maternal nutrient supply.
The study may facilitate the development of potential treatments to support fetus that suffers from maternal malnutrition or other conditions leading to insufficient nutritional transport.
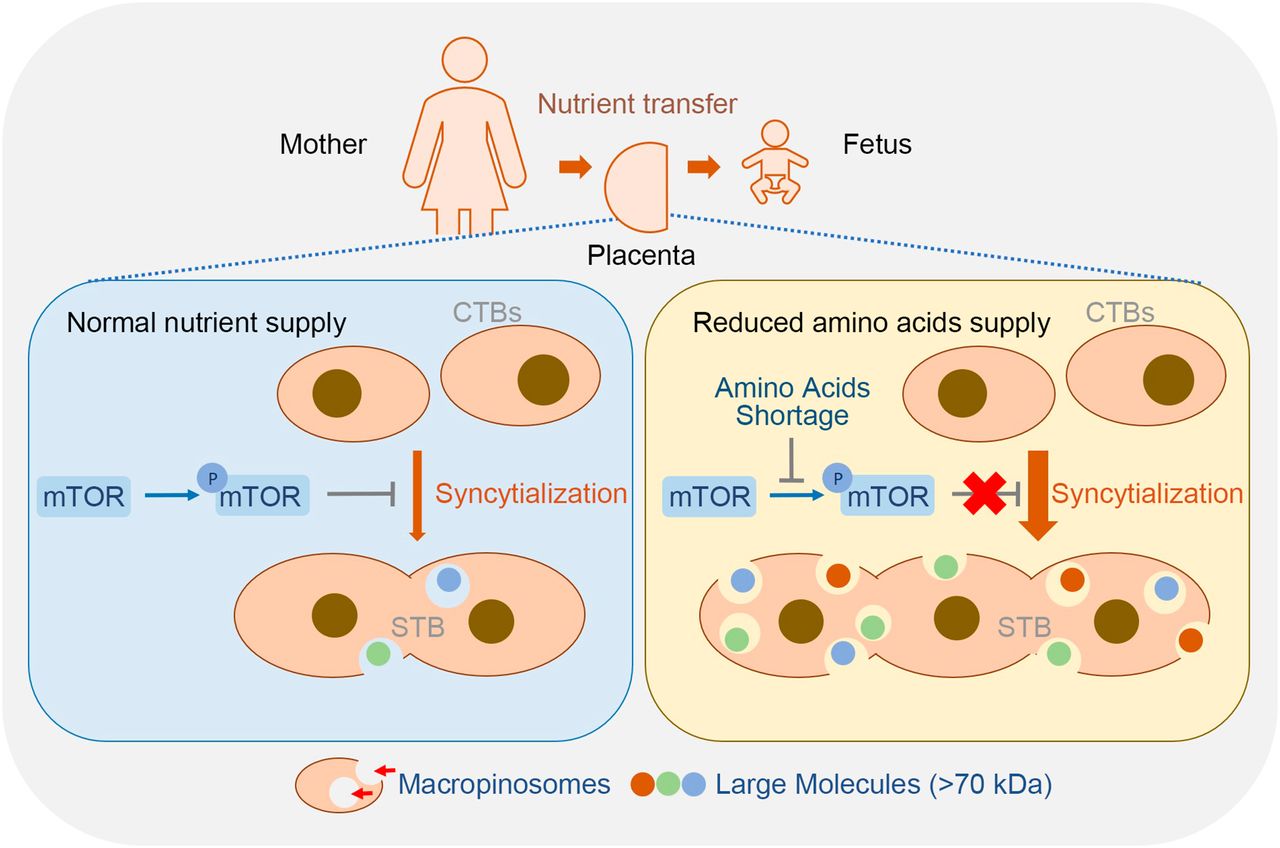
Working model of macropinocytosis-mediated adaptation to nutritional stress in placental syncytiotrophoblasts. (Image by IOZ)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

