
Newsroom
In a study published in Nature Nanotechnology, a research group led by Dr. ZHU Mingzhao from the Institute of Biophysics of the Chinese Academy of Sciences demonstrated that a dual-targeting ferritin-based nanoparticle vaccine elicits an efficient therapeutic antibody response against chronic hepatitis B.
Hepatitis B is a major global health issue caused by the infection of hepatitis B virus (HBV), which attacks the liver and can cause both acute and chronic diseases. Although conventional HBsAg vaccination has gained great success in prevention of hepatitis B virus infection, a therapeutic vaccine is still lacking.
HBV preS1 has been suggested a unique target for therapeutic vaccine development. However, due to the poor immunogenicity of preS1, a clinically translatable vaccine design that could induce efficient therapeutic antibody response is not available.
For the crucial role of lymph node (LN) antigen presenting cells (APCs) in the induction of adaptive immune responses, LN APC-targeting has been considered a valuable feature for many nanomaterial-based vaccines. In this study, the researchers found that ferritin nanoparticle (NP) based vaccine can coordinately targets SIGNR1+ resident dendritic cells and lymphatic sinus macrophages in the LNs for effective Tfh response, B cell activation, and antibody production, leading to an impressive therapeutic effect against chronic HBV infection.
According to the experimental results, in naive mice, this ferritin-preS1 NP vaccine induced robust (150-fold higher) and persistent (235 days) anti-preS1 response than control preS1 vaccine and superb memory response upon a boost immunization at day 270. The vaccination led to a significant reduction of HBV load in the peripheral blood upon AAV-HBV1.3 infection.
Furthermore, in AAV-HBV1.3 carrier mice, ferritin-preS1 NP vaccine induced a similarly high anti-preS1 response as in naive mice and significantly reduced HBV load in both peripheral blood (HBsAg, HBV DNA) and livers (HBcAg and cccDNA).
Moreover, in four out of seven mice in the ferritinNP-preS1 group, sera HBV DNA dropped to undetectable level, which also showed barely detectable HBsAg and significant amount of anti-HBs, an indicator for functional cure.
Mechanistically, the ferritin NP was first revealed to be able to target distinct SIGNR1+ myeloid cells for antibody response. While resident SIGNR1+ dendritic cells captured and processed NP antigens for Tfh induction, lymphatic sinus associated SIGNR1+ macrophages served as NP antigen depot, migrated and transferred antigens to follicular regions for B cell activation in a CXCR5-dependent manner, suggesting a novel mode of NP antigen transfer via CXCR5-dependent macrophage migration.
This study uncovers a previously unrecognized mechanism of the coordination of lymph node resident cells for antibody response engaged by ferritin NP vaccine and offers a promising translatable vaccination strategy for the functional cure of chronic hepatitis B.
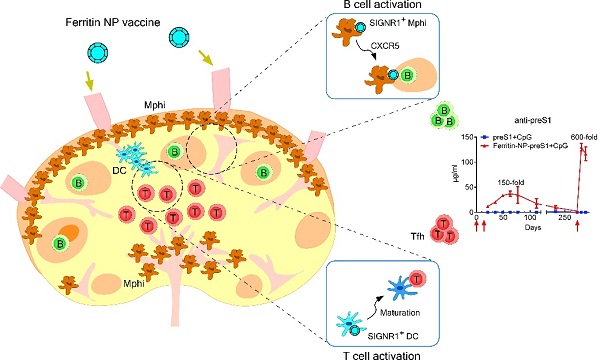
Ferritin NP vaccine coordinately targets lymph node resident SIGNR1+ macrophages and dendritic cells, induces a high-level and persistent antibody response and immune memory. (Image by Dr. ZHU Mingzhao’s group)
