
Newsroom
The functions of G-protein-coupled receptors (GPCRs) are primarily mediated by two major families of signal transducers, G proteins and arrestins. The arrestin family contains four members. Visual arrestins (Arr1 and Arr4) are exclusively involved in signal transduction in vision through binding to rhodopsin, while the non-visual arrestins (Arr2 and Arr3) are required for regulating signal transduction of many non-visual GPCRs.
Binding of Arr2 and Arr3 to GPCRs not only blocks G protein binding but also mediates receptor endocytosis and numerous G protein-independent signaling pathways. Because of the promiscuous binding and highly dynamic complex assembly, the structure determination of an arrestin complex with a non-visual GPCR is a technical challenge and a long-sought goal in the field of GPCR structural biology.
In a recent study published online in Cell Research, XU Huaqiang's group and YU Xuekui's group from Shanghai Institute of Materia Medica of the Chinese Academy of Sciences as well as the CONG Yao's group of National Center for Protein Science Shanghai revealed a complex structure of arrestin-2 bound to a G protein-coupled receptor.
XU's team firstly identified NTSR1 as the model receptor after several rounds of screening. Through eight years of systematic exploration, they developed a serial of strategies to enhance the interaction between Arr2 and NTSR1 and to improve the stability of complex.
The specific strategies include fusing the wild type human NTSR1 with the human 3A mutant Arr2 at its C-terminus, adding BRIL to the N-terminus of the receptor to increase the complex expression, co-expressing GRK5, a GPCR kinase which phosphorylated the receptor to promote arrestin binding, and fusing Fab30 and incubating with a reported PAM ML314.
Finally, they determined the structure of Arr2-NTSR1, and revealed an overall assembly that is strikingly different from the visual arrestin-rhodopsin complex by a 90° rotation of Arr2 relative to the receptor. In this new configuration, intracellular loop 3 (ICL3) and transmembrane helix 6 of the receptor are oriented toward the N-terminal domain of the arrestin, making it possible for GPCRs that lack the C-terminal tail to couple Arr2 through their ICL3.
Molecular dynamics simulation and crosslinking data further supported the assembly of the Arr2-NTSR1 complex. Sequence analysis and homology modeling suggested that the Arr2-NTSR1 complex structure may provide an alternative template for modeling arrestin-GPCR interactions.
The mechanism of visual arrestin binding to rhodopsin has been illustrated by XU Huaqiang's group with the aid of the femtosecond X-ray laser method as early as 2015.
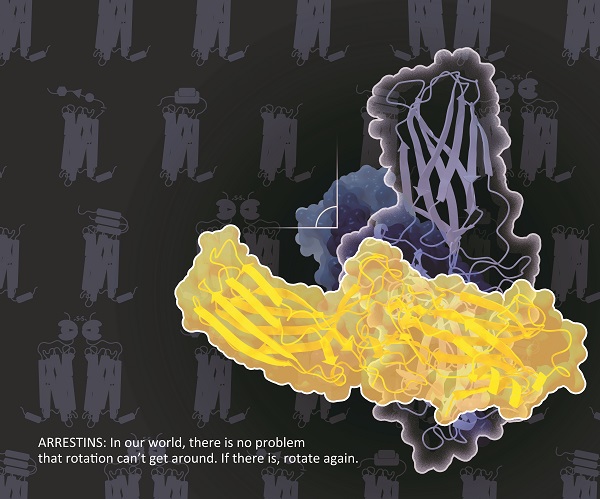
Schematic illustration of the overall assembly that is strikingly different from the visual arrestin-rhodopsin complex by a 90°rotation of Arr2 relative to the receptor. (Image by YIN Wanchao)
