Protein liquid-liquid phase separation (LLPS) can drive the assembly of various membrane-less compartments involved in a wide range of biological processes. An increasing number of proteins with diverse biological functions have been reported to undergo LLPS both in vitro and in vivo. Protein LLPS is highly sensitive to the conditions such as protein concentration, pH, ionic strength, metal ions, temperature, RNA, and crowding agent. The LLPS behavior of each individual protein is most likely distinct from that in cells. Therefore, it is important to investigate how protein LLPS is dynamically regulated in the presence of native binding partners, cofactors, PTMs, and mutations.
In a recent study published on
Cell Reports Physical Science, Prof. LIU Cong's group from the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences, together with Prof. LI Dan's group from Shanghai Jiao Tong University, has established a high-throughput protein phase separation (HiPPS) profiling method to evaluate the LLPS ability of different proteins.
According to the researchers, protein LLPS is a general property of both well-folded and unstructured proteins. More importantly, the ability of protein LLPS (termed as LLPS space) is under the regulation of protein-protein interaction, posttranslational modification, genetic mutation and chaperones.
The researchers established HiPPS contained 96 conditions based on the factors that may influence protein LLPS. During the screening process, a crystallization robot and a high-content analysis systems were used to quickly and efficiently monitor protein LLPS while minimizing the use of protein samples. Results suggest that LLPS ability of proteins is intimately contingent on conditions, and LLPS appears to be a general property of both unstructured and well-folded proteins.
Notably, multi-component co-LLPS mixture decreases the threshold concentration of individual proteins for LLPS, and LLPS space of protein can be dynamically reshaped by protein-protein interaction, posttranslational modification, genetic mutation, and cofactors.
Together, this work shows that HiPPS method may effectively characterize LLPS of individual proteins or protein mixtures in vitro, which is valuable for understanding its role in biological processes.
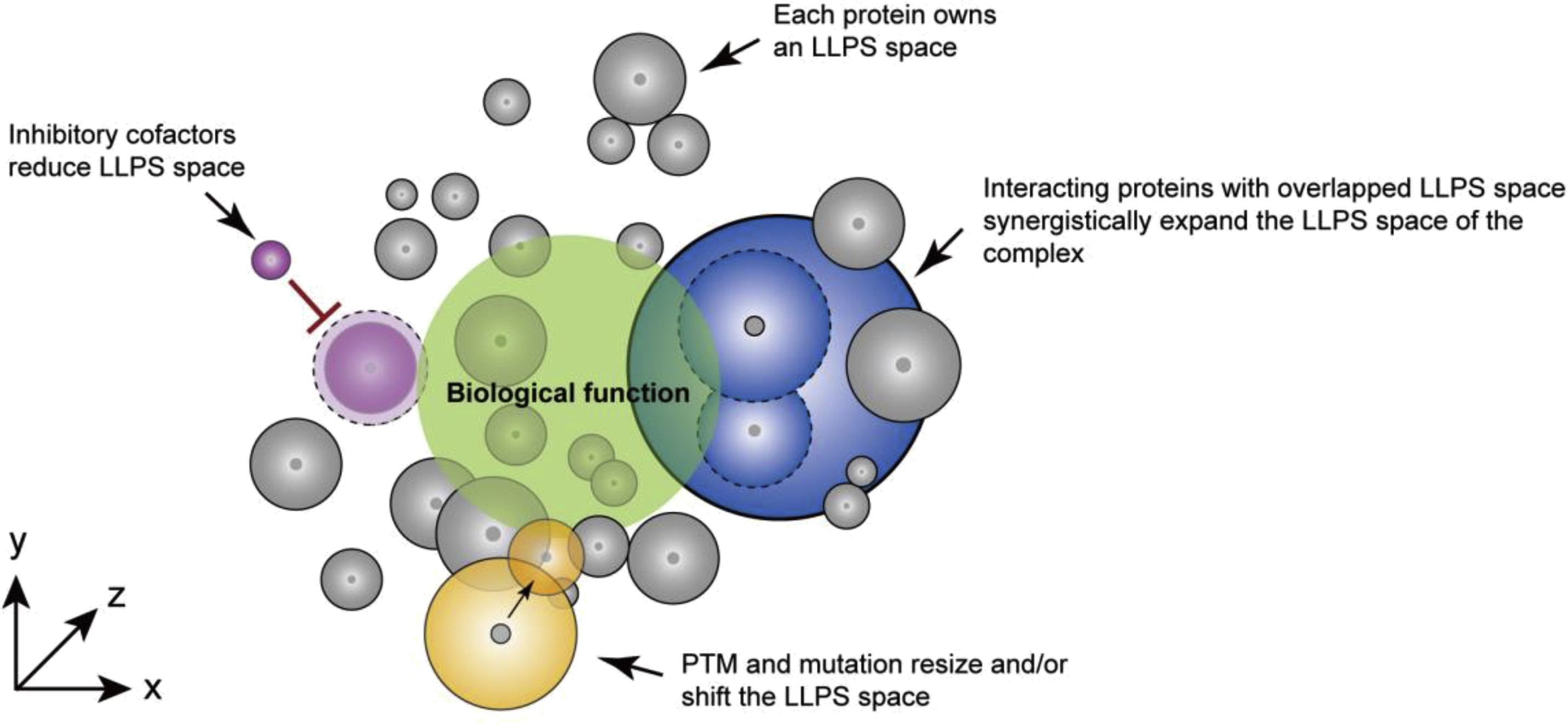
Characterization and dynamic regulation of protein LLPS space. (Image by LIU Cong)





