
The fat-mass and obesity-associated gene (FTO) was the first identified genetic factor for obesity. It plays a vital role in regulation of body weight and fat mass. However, the molecular mechanism for its regulation in obesity and the potential clinically approved drugs that can specifically inhibit its enzymatic activity are not well understood.
A study led by Dr. HUANG Niu at National Institute of Biological Sciences and Dr. YANG Yungui at Beijing Institute of Genomics of Chinese Academy of Sciences has revealed how entacapone, a FDA proved drug for Parkinson's disease, regulates energy homeostasis and body weight through inhibiting FTO’s demethylation activity on m6A. The finding was published in Science Translational Medicine.
The functional investigation of entacapone was carried out in entacapone-treatment and liver-specific FTO knockout mice.
Through a structure-based virtual screening approach, researchers discovered that entacapone can directly inhibit the demethylation activity of FTO. They observed that entacapone feeding mice exhibited some features of reprogrammed energy metabolism, such as body weight loss, lower fasting blood glucose levels and thermogenesis increase in adipose tissues. Mechanistically, transcriptome analysis revealed that G6CP, the critical gene in gluconeogenesis, was downregulated in FTO knockdown cells.
m6A is the most abundant internal mRNA modification in mRNA participating in various biological processes such as the development of mammal, immunology, stem cell renewal, and adipocyte cell differentiation. Based on the transcriptome and m6A-IP seq analyses on liver tissue isolated from liver-specific FTO conditional Knockout mice, they further demonstrated that the downregulation of FOXO1 protein in FTO conditional KO mice was due to the increased m6A level in its mRNA.
To determine how FTO regulates the expression of G6PC, researchers used adenovirus system to integrate G6PC promoter-luciferase reporter gene into the mouse liver to monitor the in vivo luciferase signal. Mice with conditional knock-out of FTO or FOXO1 in liver, or entacapone feeding displayed reduced luciferase signal, which can be restored by WT-FTO but not m6A catalytic domain mutant FTO.
These experiments illustrated the important role of FTO-FOXO1-G6PC regulatory axis in regulation of gluconeogenesis in liver.
Besides, this study revealed that the molecular mechanism of elevated thermogenesis in mouse iWAT (inguinal white adipose tissue). It showed that FTO inhibition by entacapone upregulates m6A level in FOXO1 mRNA leading to the downregulated protein abundance of FOXO1.
UCP1 promoter-luciferase assay further illustrated that the level of m6A deposited in FOXO1 mRNA affects the expression of UCP1, and FOXO1 acts as a transcription repressor inhibiting the expression of UCP1 in iWAT, which is apparently different from its function in liver.
This study elucidated the molecular mechanism through which FTO regulating gluconeogenesis in liver and thermogenesis in iWAT. The discovery of FTO inhibitor-entacapone is potentially significant in the guidance and further development of clinic drugs for obesity patients.
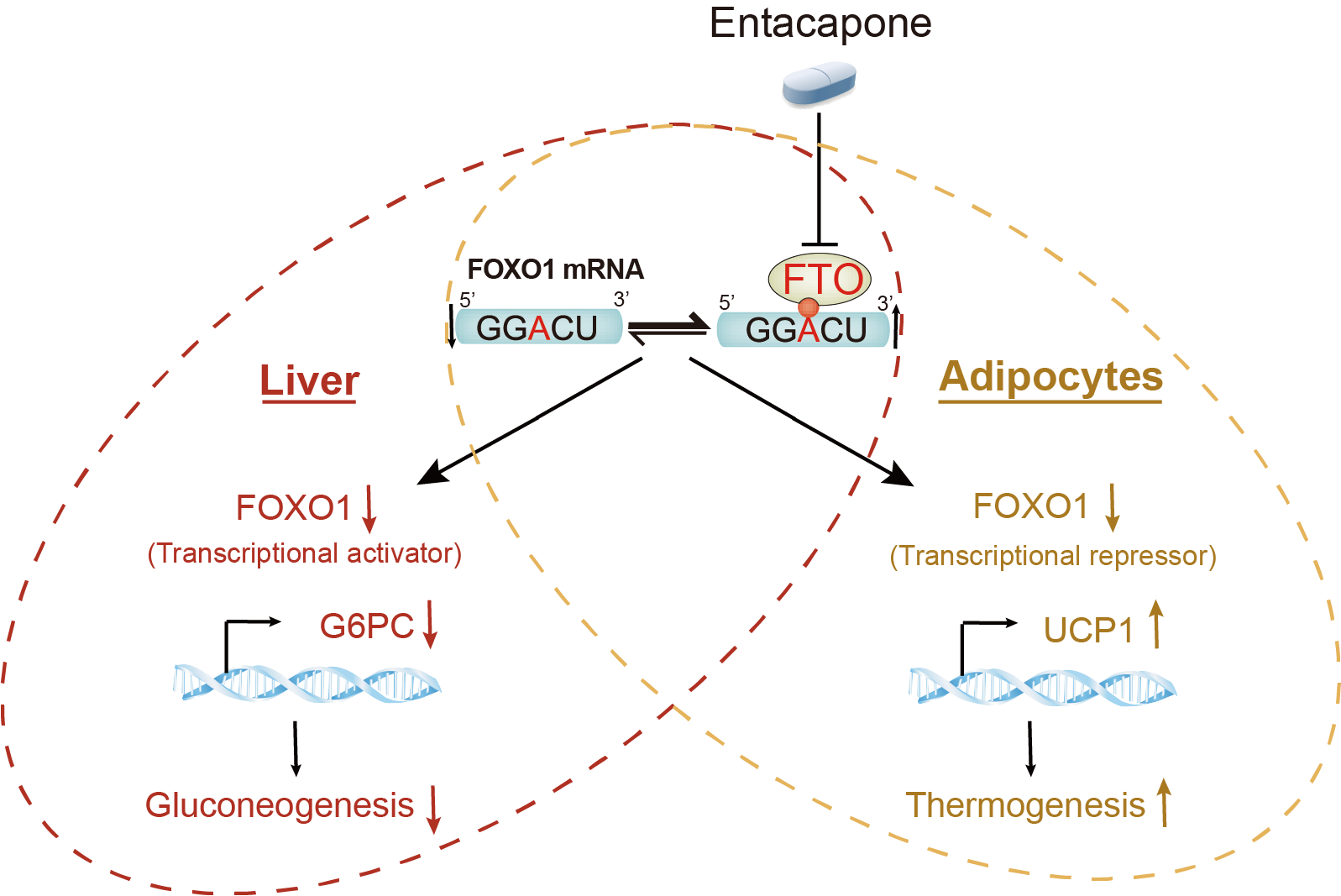
The proposed FTO-FOXO1 regulatory axis in adipose tissues and liver (Image by HUANG Niu)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

