
Phase transitions take place with heat (i.e., entropy) exchanged between materials and environments. When such processes are driven by pressure, the induced cooling effect is called barocaloric effect, which is a promising alternative to the conventional vapor compression cycle.
For the purpose of the real application, it is desirable that a material has larger entropy changes induced by smaller pressure.
Recently, an international research team led by Prof. LI Bing from the Institute of Metal Research, Chinese Academy of Sciences has found that a class of disordered materials, called plastic crystals, exhibit record-large barocaloric effects under very weak pressure.
The typical entropy changes are about several hundred joules per kilogram per kelvin, which is ten times better than the previous materials.
Utilizing large-scale facilities in Japan and Australia, the team revealed that the constituent molecules of these materials are extensively orientationally disordered on the lattices and these materials are intrinsically easy to be deformed.
As a result, a tiny pressure is able to suppress the extensive orientational disorder and thus pressure-induced entropy changes are obtained. These two merits make the plastic crystals the best barocaloric materials so far.
This research presents the first report that the entropy changes can exceed 100 joules per kilogram per kelvin and stand the best among all caloric-effect materials (barocaloric effect and its analogies such as magnetocaloric effect, electrocaloric effect, and elastocaloric effect), regarded as a milestone.
The microscopic physical scenario established using the neutron scattering technique is helpful for designing even better materials in the future.
As far as the refrigeration application is concerned, plastic crystals reported here are very promising given that they are abundantly-available, environmentally-friendly, easy-driven, and high-performance.
This work points out a new direction for the emergent solid-state refrigeration technologies.
These findings were published on Nature recently.
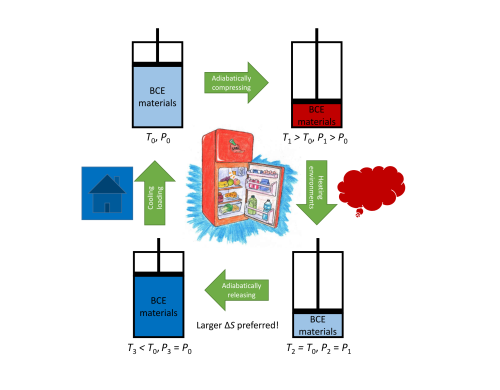
Figure 1. Schematic diagram of the refrigeration cycle based on barocaloric effects. (Image by IMR)
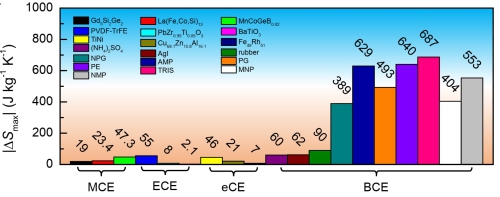
Figure 2. Comparison of the maximum entropy changes of leading caloric materials. MCE: magnetoclaoric effect; ECE: electrocaloric effect; eCE: elastocaloric effect; BCE: barocaloric effect. The plastic crystals identified in the present work are neopentylglycol (NPG), pentaglycerin (PG), pentaerythritol (PE), 2-Amino-2-methyl-1,3-propanediol (AMP), tris (hydroxymethyl) aminomethane (TRIS), 2-Methyl-2-nitro-1-propanol (MNP), 2-Nitro-2-methyl-1,3-propanediol (NMP). (Image by IMR)
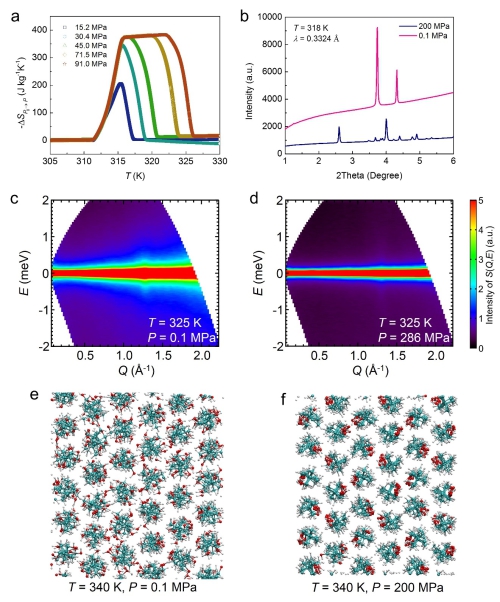
Figure 3. Pressure dependent physical properties of the representative plastic crystal NPG. (a). Pressure-induced entropy changes as a function of temperature under varying pressures. (b). X-ray diffraction patterns, showing a pressure-induced phase transition. (c,d). Neutron scattering spectra, displaying that the pressure suppresses the quasi-elastic signal originating from the molecular orientational disorder. (e,f) Structural snapshots of molecular dynamics simulations, where pressure aligns the molecules. (Image by IMR)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

