
Newsroom
Red fluorescent proteins (RFPs) that combine high brightness with strong photostability remain scarce, especially in the red spectral region, posing a major constraint on long-term live-cell super-resolution imaging and multicolor super-resolution applications.
To address this technological bottleneck, a research group led by Prof. XU Pingyong from the Institute of Biophysics of the Chinese Academy of Sciences carried out systematic protein engineering using the highly bright RFP mScarlet3 as a template.
Their work was published in Nature Methods on November 26.
The researchers first developed a photostabilized variant, mScarlet3-H. While this version exhibits moderately improved resistance to photobleaching under conventional wide-field imaging, its photostability remains inadequate for demanding super-resolution techniques, such as stimulated emission depletion (STED) and structured illumination microscopy (SIM).
The researchers then established a semi-automated screening platform for photostability and performed systematic mutagenesis of key amino acids surrounding mScarlet3-H, with particular focus on residues prone to oxidation. Through multiple rounds of combinatorial optimization, they developed a series of RFP variants with enhanced photostability, with the most optimized variant named mScarlet3-S2.
Extensive characterization revealed that mScarlet3-S2 exhibits 29-fold higher photostability compared to its template, mScarlet3, far surpassing any other known RFP. This remarkable improvement enables prolonged 2D and 3D super-resolution imaging using STED and SIM technologies.
Leveraging the exceptional photostability of mScarlet3-S2, the researchers applied it to study endoplasmic reticulum (ER)-nuclear envelope (NE) interactions, revealing numerous ultrastructural features that had not been observed previously.
The innovation of this work lies in overcoming the long-standing challenge of insufficient photostability in RFPs for super-resolution imaging. The development of mScarlet3-S2 provides a reliable red fluorescent labeling tool for long-term live-cell imaging and, through its outstanding performance in STED and SIM, expands researchers' understanding of subcellular structural complexity.
This advancement is expected to drive progress across multiple fields of cell biology, particularly for long-term, high-resolution observation of organelle dynamics, membrane contact site functions, and intracellular transport processes. As mScarlet3-S2 becomes widely adopted in scientific research, it is anticipated to enable numerous unprecedented biological discoveries in live-cell super-resolution imaging.
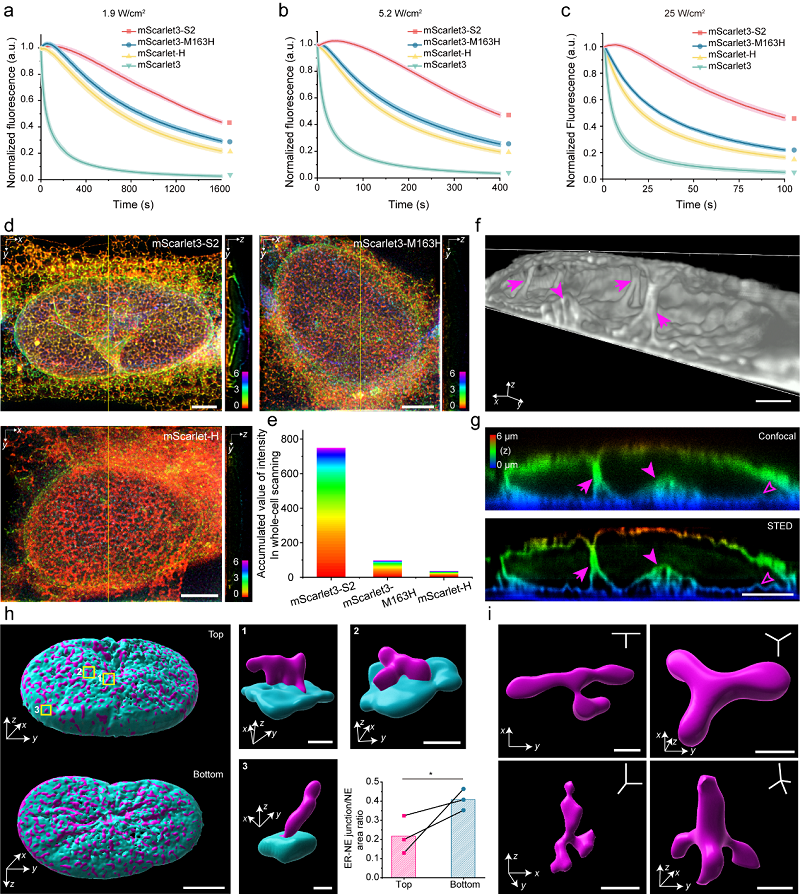
a-c. Photostability of mScarlet3-S2 under different energy density conditions; d-e. 3D STED imaging of the endoplasmic reticulum labeled with different RFPs; f-g. Perinuclear ER structures invaginating into the nucleus; h. Spatial distribution map of ER-nuclear envelope contact sites; i. Planar and nonplanar three-way and four-way ER junction structures. (Image by XU Pingyong's group)
