
Newsroom
Carbon-center radicals have garnered interest due to their distinctive electrical, optical, and magnetic properties deriving from the unpaired single electron. Up to now the construction of radical materials has been limited to the complex design and prolonged synthesis of radical building blocks.
Hydrogen-bonded organic frameworks (HOFs), typically characterized by monomers interconnected through hydrogen bonds and π-π stacking, have emerged as potential radical materials.
In a study published in Chem, a team led by Prof. LIU Tianfu from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences reported a novel air-stable radical HOF, PFC-1-R, as a typical n-type semiconductor. The stability of PFC-1-R could be improved through the pore engineering strategies, including modulatory arrangement of monomers and close-packed structure.
Researchers introduced a facile and effective strategy for the construction of air-stable radical HOFs through post-synthetic oxidation. The porous nature of the HOFs allowed efficient permeation of oxidants into the channels, enabling adequate radical production. These distinctive radicals could be synthesized exclusively by solid-state reactions within HOFs, providing a novel perspective for fabricating unconventional radicals inaccessible through solution-based approaches.
Besides, the extension of spin delocalization over the conjugated backbone contributed to the longevity of the radicals, resulting in exceptional radical stability in air and different solvents. The open-shell electronic structure imparted distinctive physicochemical properties to these materials, such as improved conductivity, near-infrared photothermal capacity, and paramagnetism. This strategy demonstrated HOFs' applicability, revealing the potential for fabricating stable radical HOF materials with unprecedented properties.
Experimental results indicated that the PFC-1-R exhibited high radical concentrations with paramagnetic behavior equivalent to two monomers sharing an unpaired electron. The HOF could maintain their radical nature under ambient conditions on account of spin delocalization along the [π···π] stacked units. The radical formation transformed the HOFs from insulators to typical n-type semiconductors.
This study not only presents a facile and low-cost method for constructing air-stable radical HOF materials but also highlights the pivotal role that reticular chemistry can play in advancing radical semiconductors.
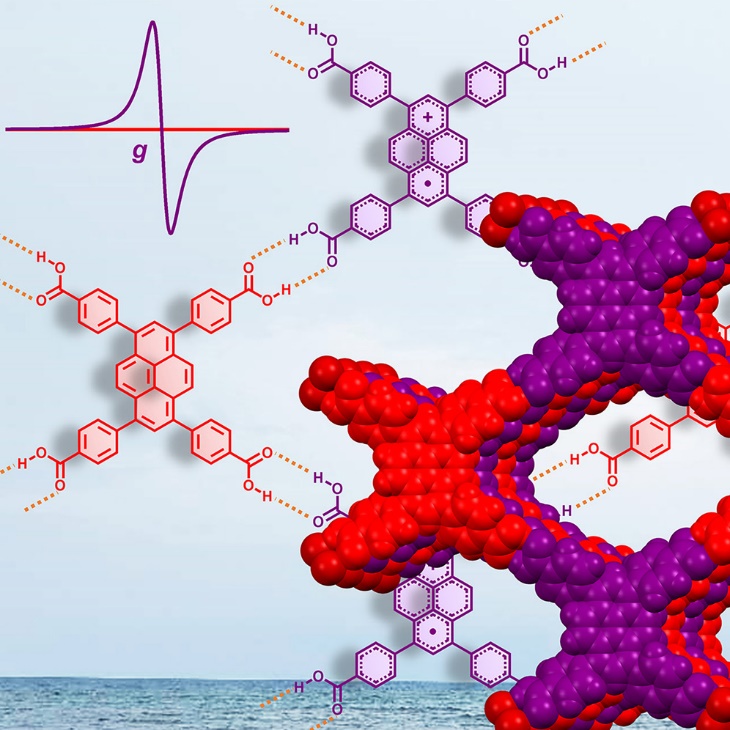
PFC-1-R exhibits high radical concentrations with paramagnetic behavior equivalent to two monomers sharing an unpaired electron. (Image by Prof. LIU’s group)
