
Newsroom
Synthesis of liquid fuel (C5+ hydrocarbons) via CO2 hydrogenation is generally accelerated by heterogeneous catalysts, which involves cascade catalysis of reverse water gas shift (RWGS) reaction to generate CO, and subsequent CO hydrogenation to hydrocarbons via Fischer-Tropsch synthesis (FTS). However, RWGS reaction is endothermic and needs higher temperature, whereas FTS reaction is exothermic and higher temperature tends to inhibit the reaction selectivity.
The homogeneous catalysts are well known for their high efficiency at low reaction temperature. If the RWGS reaction is accelerated effectively at low temperature by a homogeneous catalyst, the thermodynamic limitations may be overcome. The thermodynamic limitations usually result in high reaction temperature (above 300 oC) and low selectivity of C5+ hydrocarbons.
Inspired by this, Prof. HAN Buxing and Prof. QIAN Qingli from the Institute of Chemistry of the Chinese Academy of Sciences and the collaborators combined homogeneous and heterogeneous catalysis in the production of liquid fuel from CO2 and H2, and achieved excellent results at much lower temperature. The study was published in Chem,
The reaction could be effectively catalyzed by homogeneous RuCl3 and heterogeneous Ru0 catalysts in 1-methyl-2-pyrrolidinone (NMP) solvent, where LiCl and LiI were utilized as cocatalyst and promoter, respectively. It was conducted at 180 oC, which is much lower than those reported in the literature.
The selectivity of liquid hydrocarbons (C5-C28 n-paraffins) could reach 71.1%, which is the highest to date. The turnover frequency (TOF) of the reaction was as high as 9.5 h-1, which is comparable to the best level of the FTS reaction using Ru0 catalyst. Moreover, the products were all n-paraffins, which has not been reported before in liquid fuel synthesis via CO2 hydrogenation.
The control tests demonstrated that the homogeneous RuCl3 catalyst and the heterogeneous Ru0 catalyst could be recycled and reused, respectively. This suggested that both of them had good stability when they are utilized separately.
When they are coupled together in a reactor, the contents of the homogeneous catalytic components (cationic Ru, Li+, Cl- and I-) before and after the reaction were nearly the same, which confirmed the stability of the homogeneous and the heterogeneous catalysts in the consecutive reactions.
Further research indicated that synergy of catalytic components and/or the consecutive reactions accounted for the outstanding performance of the catalytic system.
This study provides a new way for high-efficient production of liquid fuel via CO2 hydrogenation at milder condition.
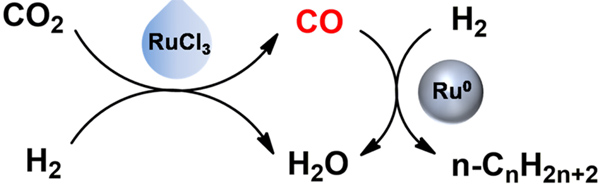
Hydrogenation of CO2 by coupling homogeneous and heterogeneous catalysis (Image by Prof. HAN Buxing)
