
The Neural Synaptic Mechanism and Function Group led by Dr. SHENG Nengyin at Kunming Institute of Zoology of Chinese Academy of Sciences, collaborating with Dr. Roger Nicoll Lab at University of California, San Francisco, set up the conditional knock-out mice of all the three genes encoding AMPARs as the model system, and revealed the postsynaptic molecular mechanism underlying synaptic plasticity LTP. The study was published in PNAS.
The researchers constructed a tethered AMPAR of GluA1 receptor with its auxiliary subunit transmembrane AMPA receptor regulatory protein (TARP) g-8, named GluA1-g-8, and then replaced the endogenous AMPARs through in utero electroporation, thereby to study the function and mechanism of the interaction of glutamate receptor complex and PDZ domain-contained postsynaptic scaffolding proteins during LTP.
Combining the techniques of hippocampal slice culture and electrophysiology, as well as many others, they found that the trafficking of exogenous expressed AMPARs is only regulated by the tethered g-8 protein. Moreover, the PDZ-binding site-mediated interaction, between AMPAR/TARP receptor complex and postsynaptic scaffolding proteins, is necessary for the synaptic basal transmission and LTP expression of this receptor complex.
Further study demonstrated that the interaction with the PDZ-domain of postsynaptic scaffolding proteins is also required for LTP of the receptor complex of kainite receptor (KAR) with its auxiliary subunits Neto proteins, another subfamily member of glutamate receptor.
This study suggested that the postysynaptic mechanism underlying LTP is conserved and regulated by a general mechanism, regardless of the subtype of glutamate receptors, and the PDZ domain-contained scaffolding proteins are the targets for modification during LTP, whereas the glutamate receptor/auxiliary subunit complexes play passive roles. It provided theoretical basis for learning and memory study, and the pathological mechanism of related mental disorders.
This work was funded by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences, National Natural Science Foundation of China and the Chinese Academy of Sciences Pioneer Hundred Talents Program.
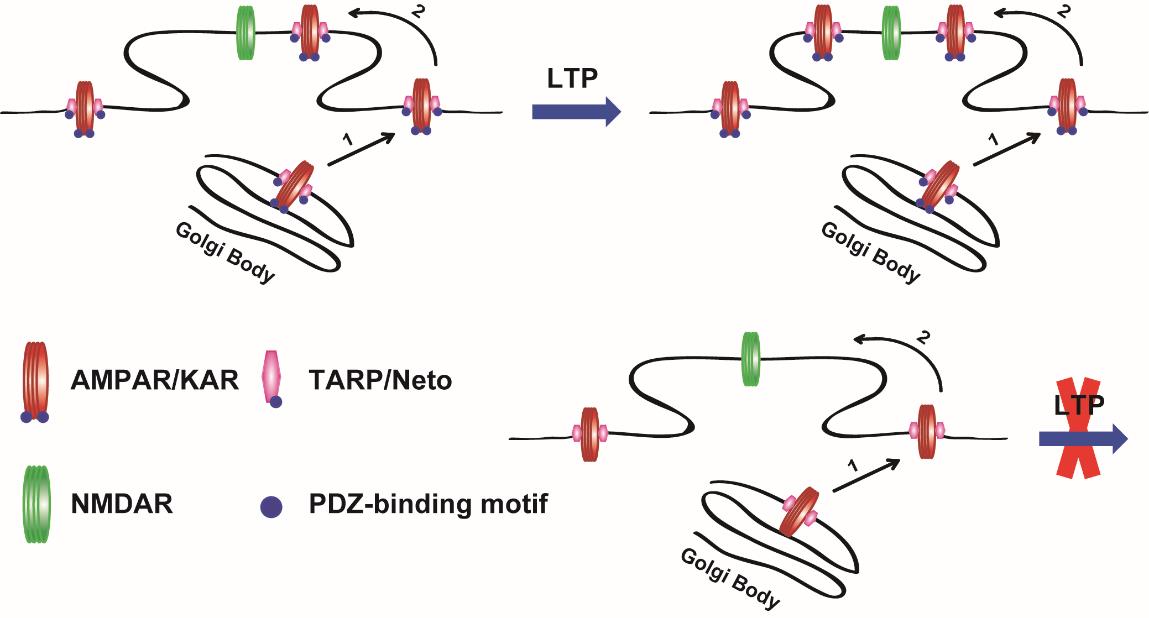
Working Model of postsynaptic PDZ-domain interactions with glutamate receptor/auxiliary protein complexes for LTP expression. (Image by Dr. SHENG Nengyin’s Group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

