
The pervasive pollution of organic micropollutants sand fluoride in water resources causes damage to aquatic ecosystems and human health. Therefore, it is of great importance to develop efficient and cost-effective adsorbents to remove fluoride and organic micropollutants from contaminated water.
To address this problem, LIU Jinhuai and KONG Lingtao's joint team from Institute of Intelligent Machines, Hefei Institutes of Physical Science, Chinese Academy of Sciences, successfully prepared a new kind of adsorbent to remove fluoride and organic micropollutants for the first time.
In their work, they demonstrated the novel ultralong hydroxyapatite (HAP) nanowires as effective and biocompatible adsorbents to remove fluoride and organic micropollutants from contaminated water.
According to their report, it was the first time they managed to prepare novel ultralong hydroxyapatite (HAP) nanowires and Al(OH)3 nanoparticles modified hydroxyapatite (Al-HAP) nanowires for fluoride removal.
The maximum of adsorption capacity was 40.65 and 93.84 mg/g for HAP and Al-HAP. The thermodynamic parameters suggested that the adsorption of fluoride was a spontaneous endothermic process. Both anion excxrhange and electrostatic interactions were involved in the adsorption of fluoride.
Furthermore, the HAP and Al-HAP were made into filter membrane. Membrane filtration experiments revealed that the high fluoride removal capabilities on HAP and Al-HAP membranes depended on the membrane thickness, flow rate and initial concentration of fluoride. The effectiveness of as-synthesized HAP and Al-HAP for fluoride removal from contaminated water was thus verified.
Besides, the HAP nanowires were made into adsorption membranes with a sandwich structure that was developed to remove organic micropollutants for the first time. The hydrophilicity and hydrophobicity of the HAP adsorbent and membrane were tunable by controlling the surface structure of HAP.
The HAP membrane could remove organic micropollutants effectively by dynamic adsorption in both aqueous and ethanol solutions. In the adsorption process, the removal efficiencies of organic micropollutants depended on the solution composition, membrane thickness and hydrophilicity, flow rate, and the initial concentration of organic micropollutants.
The adsorption capacities of the HAP membrane with a sandwich structure were at a high level. The biocompatible HAP adsorption membrane can be easily regenerated by methanol.
These results demonstrated that the HAP and Al-HAP could be a novel concept to remove fluoride and organic micropollutants from both aqueous and organic solutions.
This work was supported by the National Key Scientific Program-Nanoscience and Nanotechnology and the National Natural Science Foundation of China.
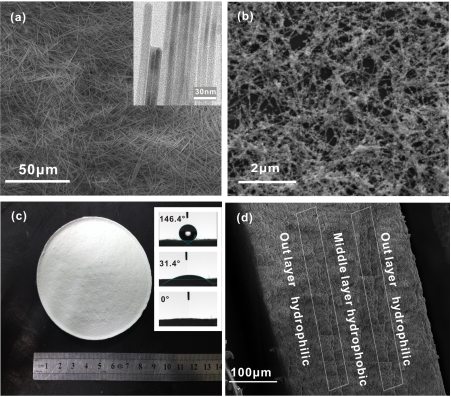
Fig. 1. (a) SEM and TEM images of the HAP nanowires; (b) SEM image of the Al-HAP nanowires; (c) Optics image and contact angles of the HAP membrane; (d) The SEM image of cross section for the HAP membrane with a sandwich structure. (Image by HE Junyong)
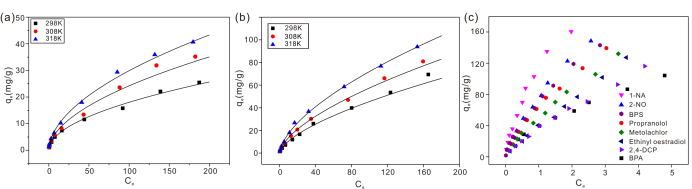
Fig. 2. (a) Fluoride adsorption isotherms on HAP nanowires at three different temperatures; (b) Fluoride adsorption isotherms on Al-HAP nanowires at three different temperatures; (c) Adsorption isotherms of the eight organic micropollutants on HAP nanowires. (Image by HE Junyong)
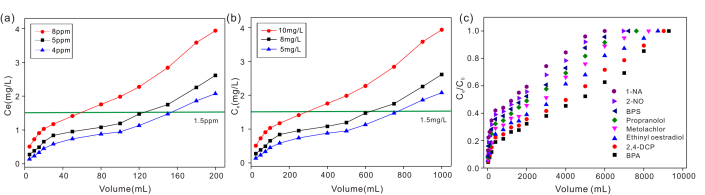
Fig. 3. (a) Adsorption behaviour of fluoride by HAP membrane; (b) Adsorption behaviour of fluoride by Al-HAP membrane; (c) Adsorption behaviour of different organic micropollutants by HAP membrane. (Image by HE Junyong)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

