
Cyclic compounds hold a vital position in modern organic chemistry with their ubiquity in nature and serve as one of the most important materials in both academia and industry. Thus the synthesis of cyclic compounds is extremely important in organic chemistry. To date, chemists have plentiful methods for generating small rings with six or fewer members and constructing large rings with ten or more members. However, the synthesis of medium-sized rings, especially catalytic asymmetric variants, remains a great challenge because of their unfavorable transannular interactions and entropic factors.
A research group led by YOU Shuli at Shanghai Institute of Organic Chemistry of Chinese Academy of Sciences recently developed a novel strategy for enantioselective synthesis of indole-annulated medium-sized-ring compounds (J. Am. Chem. Soc.). This study has been highlighted by Chemical & Engineering News with the title "Asymmetric third ring is a charm for indole-annulated compounds" on May 9, 2016.
The nucleophilic attack of the indole C3 position on the π-allyliridium moiety delivers the bridged intermediate II. The reaction is feasible because the substrates are pre-organized by a six- or seven-membered-ring to reduce energetically unfavorable transannular and torsional strain. Subsequently, a ring-opening retro-Mannich reaction affords the ring-expansion intermediate III, from which hydrolysis of the iminium moiety gives the desired product. The proposed mechanism is supported by in situ reduction of the bridged cyclic intermediate. Under mild reaction conditions, various seven-, eight-, or nine-membered rings can be formed smoothly. This novel synthetic strategy opens a window for asymmetric synthesis of medium-sized rings.
YOU's group has been committed to the study of transition-metal-catalyzed asymmetric allylic dearomatization reactions (Acc. Chem. Res.2014,47, 2558) for many years. In 2013, they developed a highly efficient synthesis of enantioenriched polycyclic indoles and pyrroles via a dearomatized spiro intermediate through an in situ migration pathway (J. Am. Chem. Soc. 2013,135, 8169). In 2015, they reported a highly diastereo- and enantioselective synthesis of spiroindolines by a dearomatization strategy (Angew. Chem., Int. Ed.2015, 54, 14146.). Furthermore, a chiral tryptamine derivative was easily constructed in good yield and with excellent ee by retro-Mannich/hydrolysis cascade reaction of the spiroindolenine intermediate when an aromatic substituent was introduced at the benzylic position of the indole substrate.
These projects were sponsored by the National Natural Science Foundation of China, Ministry of Science and Technology, Chinese Academy of Sciences, and Science and Technology Commission of Shanghai Municipality.
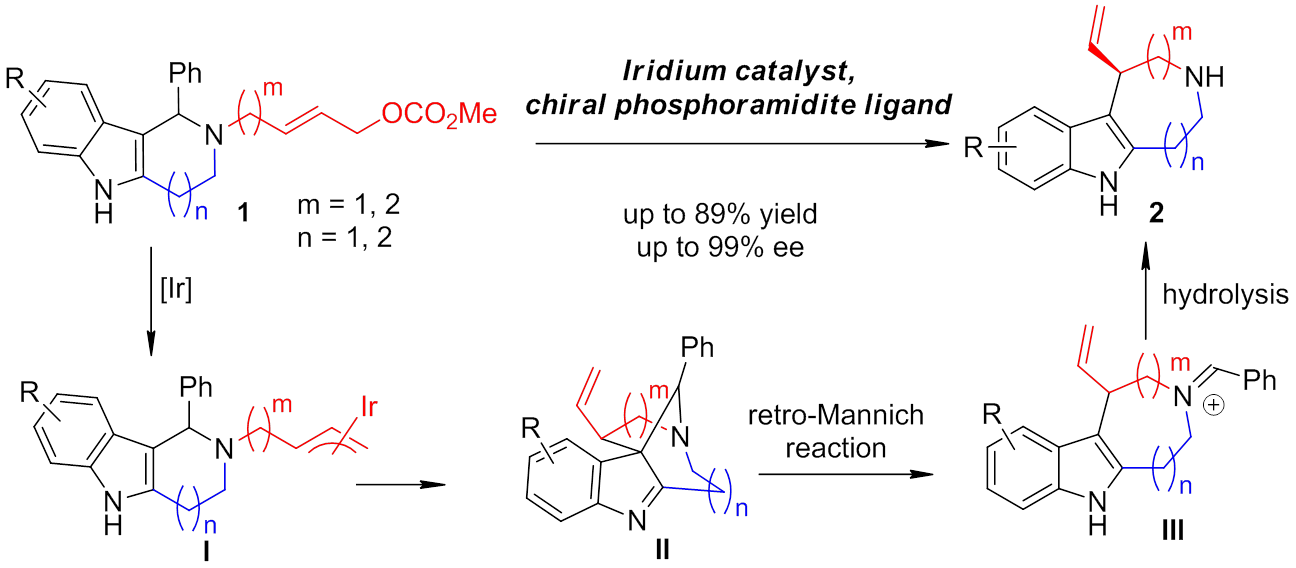
A novel strategy for enantioselective synthesis of indole-annulated medium-sized-ring compounds. (Image by YOU Shuli)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

