
When staring at the starry sky, we are amazed by the magnificence of the cosmos, wondering how many secrets are hidden in that boundless deep space. When we look back at ourselves, we are even more amazed by another complex and mysterious cosmos hidden inside each of us: our brain. Our brain consists of approximately 100 billion neurons, which is about ten times the number of the stars in the galaxy, and each neuron makes on average 1000 connections, known as "synapses”, with other neurons.
At all time, through these synapses, neurons in the brain receive neural inputs carrying different forms of information from other neurons in the network, integrate and process them, and then send them out to other neurons in the network. Thus, synapses connect neurons into thousands of neural circuits, which are further connected into the enormous and complex neural network that is the brain. Stimuli from the external world, including images, sounds, odors, and textures of objects are received by the eyes, ears, noses and fingers and converted into electrical and chemical signals. These signals rush all the way through neural circuits into the brain to generate the sensations of seeing, hearing, olfaction and touch. In other words, when people respond to these external stimuli, the neural signals carrying the “command” messages rush from the brain to every corner of the body, which generates actions and effects, voluntarily or involuntarily.
In fact, the interplay between the biological brain and the external world is far more complex than simple exchange of information. It has been recognized that the brain is very “plastic”, because many of its functions can be altered in response to changes in external stimuli. For example, people can learn to distinguish colors, shapes, odors or textures and learn skills such as speaking a foreign language, riding a bicycle or playing the piano. The study of these skills often best occur within a “sensitive period”, a developmental period during which neurons are particularly sensitive to modification by experience, after which learning becomes less effective, or not possible at all. The plasticity of the brain implicates that connections between neurons in the brain, responsive for sensation, motion, memory, emotion and even possibly personality, are not fixed but rather refined and rewired by external stimuli throughout life.
As parents would find, identical twins having same genes develop into different individuals with distinct personalities. It is hard to imagine that the neural circuits within their brains are wired differently. Besides, it is the distinct life experience that shapes people into unique individuals. How does the shaping happen? Or how do external stimuli regulate the forming and refinement of neural circuits in the neuroscience jargon? This is a question remains largely unanswered.
The transmission of information between neurons is achieved through synapses, which serve as the basic connecting points of neural circuits. The majority of excitatory synapses are located in the dendrite spines of neurons and consist of two parts: the presynaptic axon terminus that sends out information and the postsynaptic dendritic spine that receives it. Dendritic spines, or for short, spines, are tiny, thorn-like protrusions extending from dendrites, originally recorded in the exquisite drawings of Santiago Ramón y Cajal, a Spanish neuroanatomist and the father of modern neuroscience (Figure 1).
By counting the number of spines and analyzing their shapes, neuroscientists estimate the total number of synapses within a neural circuit and the strength of these synapses. Previous studies show that the number of spines rapidly increases in the early life, suggesting extensive formation of connections between neurons. However, during the transition through adolescence, spine numbers decrease significantly, suggesting that existing connections are pruned to refine the overall circuit (Figure 2). This spine pruning process is believed to play a critical role in normal functioning of the brain, and spine pruning defects have been implicated in developmental neurological disorders including autism spectrum disorders and schizophrenia. The molecular control underlying this process is poorly understood.
Whiskers are important and well-developed sensory organs in mice. Sensory inputs through whiskers play critical roles in food seeking, exploration, danger sensing and general daily life of mice. Dr. YU Xiang’s research group at the Institute of Neuroscience, Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences used the barrel field of the mouse somatosensory cortex (S1BF), which receives sensory inputs from the whiskers, as a model system to study the molecular mechanism underlying spine pruning (Figure 3).
The researchers found the spine pruning in this region is coordinated with spine maturation and bidirectionally regulated by sensory experience, i.e. both processes were accelerated by enriched sensory activity and blocked under whisker deprivation. Their hypothesis based on these observations that the pruning and maturation of different spines are the outcomes of the same competition event: neighboring spines compete for a certain limited molecular resource, with the winner surviving and maturing, at the expense of the loser being pruned. This study entitled “Coordinated spine pruning and maturation mediated by inter-spine competition for cadherin/catenin complexes” was published on Cell.
A unique protein complex, called the cadherin/catenin cell adhesion complex, came into researchers' eyes. Cadherin/catenin complexes are located at synapses, directly surrounding pre-synaptic neural transmitter release sites and post-synaptic receptors. These complexes have three major components. Transmembrane N-cadherins stretch out their extracellular domains to bind presynaptic N-cadherins like molecular zippers and achieve the “adhesive” effect. Inside the cell membrane, β-catenins, αN-catenins and the intracellular domains of N-cadherins form a complex to recruit the actin cytoskeleton, thereby providing physical support for the spine structure. N-cadherins undergo active membrane trafficking and/or endocytosis in response to changes in neural activity, thereby linking pre-synaptic changes of neural activity with post-synaptic cytoskeletal changes in spines.
Dr. YU’s group performed live imaging in cultured hippocampal neurons and found that locally enriching cadherin/catenin complexes on a single spine simultaneously induced the enlargement of the manipulated spine and the shrinkage/elimination of a neighboring spine. Similar spine fate differentiation can also be induced by activating a spine but not its neighbor using optogenetic methods which leads to the enlargement of the active spine at the expense of its neighbor. This fate differentiation depends on the redistribution of cadherin/catenin complexes between the two spines, the inter-spine distance and N-cadherin motility, but not on protein synthesis or degradation. The findings further support the competition-based model.
To verify the competition-based model in vivo, Dr. YU’s group combined mouse genetics with viral tools to individually increase cadherin/catenin-dependent adhesion in a subset of spines in S1BF at the onset of the pruning period. They found that these manipulated spines tended to survive the pruning process while their neighboring spines were more likely to be pruned. Further experiments demonstrated that regulation of spine pruning and maturation by neural activity also relies on the cadherin/catenin complex.
Dr. YU’s group is one step closer to a complete understanding of brain plasticity. In the process of neural circuit refinement during adolescence, a limited molecular resource known as the cadherin/catenin complex plays a critical role. These complexes stabilize dendritic spines by strengthening adhesion between them and their pre-synaptic counterparts, the axon termini. The normal level of the cadherin/catenin complex inside a neuron is not enough to go around every spine, naturally forcing neighboring spines to compete for it. Those receiving stronger activity inputs are more likely to win the competition and grab more cadherins and catenins to form adhesion complexes. Once the initial superiority is established, more and more cadherin/catenin molecules flow into the winner spine likely via positive feedback, and the loser spine keeps losing these molecules that are essential to its structure, a loss which inevitably leads to its collapse. Thus the loser spine is left no choice but to embrace the fate of being pruned in the end.
The nervous system uses this mechanism of competition to ensure that more active neural circuits are retained and strengthened, and the less active ones pruned. The utilization of biological resources is optimized and neural circuits are refined. This inter-spine competition mechanism based on the limited access of molecular resources echoes the basic principle of “use it or lose it” in neuroscience at the subcellular level. It expands our knowledge of neural circuit refinement and brain plasticity and represents a principal strategy employed by biological systems.

Figure 1: Spanish neurologist Santiago Ramóny Cajal (1852~1934) and his drawings of cerebellar Purkinje neurons and dendritic spines. (Image by YU Xiang)
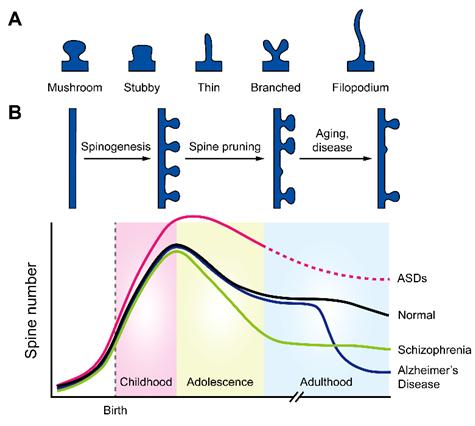
Figure 2: (A) Schematics showing four spine subtypes and filopodium. (B) Dynamics of spine number in normal development (black) or under autism spectrum disorder (pink), schizophrenia (green), or Alzheimer’s disease (blue). (Image by YU Xiang)
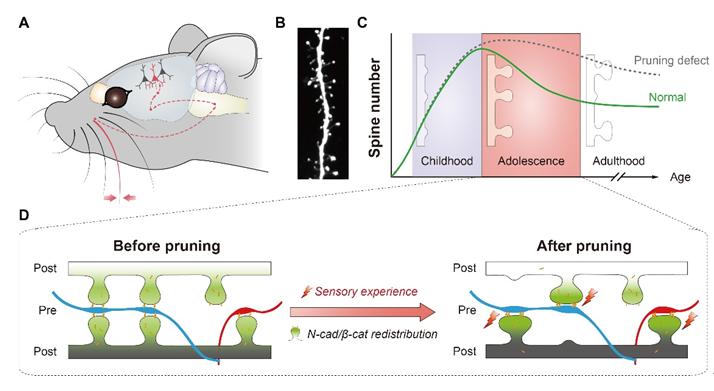
Figure 3: A model system to study the molecular mechanism underlying spine pruning. (Image by YU Xiang)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

