
Generally, transition metal catalysts are essential for reductive hydrogen atom transfer reaction, also known as transfer hydrogenation reaction or the borrowing-hydrogen reaction. It has been reported that graphene can be an active catalyst in ethylene and nitrobenzene reductions, but no report has described carbon-based materials as catalysts for alcohol amination via the borrowing-hydrogen reaction mechanism.
The group headed by Prof. SHI Feng at the Lanzhou Institute of Chemical Physics (LICP) of the Chinese Academy of Sciences has realized the transition metal-free reductive hydrogen atom transfer transformation, using a carbon material as catalyst with alcohol amination as well as nitro compound and ketone reduction as model reactions.
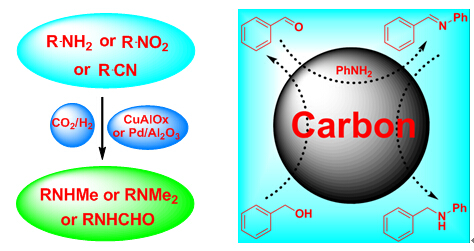
The carbon catalyst exhibited excellent catalytic performance and good generality in the above reactions. The catalyst/product separation was easily performed, and the carbon catalyst was recyclable for several runs without observable deactivation. The results suggested that C=O group may be the catalytically active site in this catalyst, while a high surface area of carbon catalyst is important for achieving high activity. The activity of the base-activated carbon catalyst remained unchanged during the reuse process due to its stable structure.
This new catalytic system may provide an attractive and useful methodology for a wider range of applications for industrially important compounds and intermediates.
The work has been published in Nature Communications(2015, 6, 6478, doi: 10.1038/ncomms7478) .

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

