
Research in Prof XU Tao's lab focuses on the molecular mechanisms of vesicle transport and fusion in pancreatic beta cells and fat cells, with the aim of elucidating the molecular and cellular mechanisms underlying blood glucose regulation. The processes of insulin secretion and fusion of glucose transporter-containing vesicles with the plasma membrane are two important events which are directly related to blood glucose regulation. Glucose uptake in fat and muscle cells occurs mainly via glucose transporter 4 (GLUT4) which translocates from intracellular compartments to the cell surface in response to insulin stimulation. This process involves sequential activation of PI3K and Akt after insulin binds to its cell surface receptor. AS160 is a substrate of Akt that is known to play a critical role in insulin-regulated GLUT4 translocation, possibly by negatively regulating the activity of Rab protein(s) involved in GLUT4 translocation.
In their recent Cell Research paper1, Prof Xu's group used mammalian tandem affinity purification (TAP) combined with mass spectrometry to identify novel partners that interact with AS160 and are involved in GLUT4 trafficking. They identified a new AS160-binding protein called RuvB-like protein 2 (RUVBL2), which is a member of a highly conserved protein family and is homologous to bacterial RuvB. RUVBL2 is known to have roles in the regulation of gene expression, cell development, and glucose metabolism. Here Prof Xu's group present evidence that the role of RUVBL2 is not restricted to the regulation of transcription in the nucleus, but is also involved in insulin-stimulated GLUT4 trafficking in the cytosol. They showed that RUVBL2 is highly expressed in 3T3-L1 adipocytes, mainly being distributed in the cytosol, and confirmed its involvement in insulin-stimulated GLUT4 translocation by knock-down of RUVBL2 in these adipocytes. Depletion of RUVBL2 inhibited insulin-stimulated GLUT4 translocation and glucose uptake by reducing insulin-stimulated AS160 phosphorylation, but these effects could be reversed by the introduction of human RUVBL2. While the precise mechanism of RUVBL2's effect on AS160 phosphorylation remains to be elucidated, these findings provide novel insights into the functions of AS160.
Financial support for this work came from the National Natural Science Foundation (Grant No. 30630020), the National Basic Research Program (Grant No. 2004CB720000), and the Chinese Academy of Sciences (Grant No. KSCX1-YW-02-1).
1 Xiangyang Xie, Yu Chen, Peng Xue, Yong Fan, Yongqiang Deng, Gong Peng, Fuquan Yang, Tao Xu (2009) RUVBL2, a novel AS160-binding protein, regulates insulin-stimulated GLUT4 translocation. Cell Research 19:1090-1097. doi: 10.1038/cr.2009.68
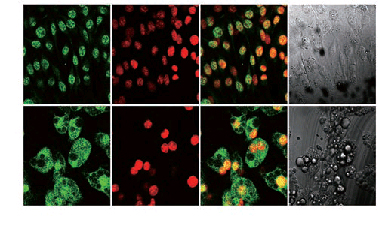
Figure Subcellular localization of RUVBL2 in 3T3-L1 fibroblasts and adipocytes. 3T3-L1 fibroblasts and adipocytes were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton. RUVBL2 was stained with anti-RUVBL2 antibody and FITC-conjugated goat anti-rabbit secondary antibody, and the cellular nucleus was stained with DAPI. Results presented are representative of three independent experiments.

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

