
A study of protein activity in autophagy was recently published online by scientists from the Institute of Biophysics (IBP) of the Chinese Academy of Sciences.
The research, entitled Structural Basis of the Differential Function of the Two C. Elegans Atg8 Homologs, LGG-1 and LGG-2, in Autophagy, appeared Dec. 17 in Molecular Cell. The work was conducted by scientists from the National Laboratory of Biomacromolecules of IBP under the direction of Prof. ZHANG Hong.
Autophagy, which can selectively recognize and remove protein aggregates, involves the engulfment of a portion of the cytosolic contents in a double-membrane autophagosome and its subsequent delivery to the lysosome for degradation. The ubiquitin-like protein Atg8, which is conjugated to phosphatidylethanolamine (PE), acts at multiple steps of autophagosome formation by directly interacting with other ATG proteins. Atg8-PE also interacts with autophagy receptor proteins (e.g., p62 and NBR1) to confer cargo selectivity in selective autophagy.
The complexity of the autophagic machinery in higher eukaryotes is conferred by the presence of multiple homologs of the same Atg proteins and also by the involvement of metazoan-specific autophagy genes such as the EPG genes previously identified in Prof. ZHANG’s laboratory. There are at least seven Atg8 homologs belonging to the LC3 and GABARAP/GATE-16 subfamilies in mammals.
However, the different functions of distinct Atg8 homologs during animal development and the mechanisms by which cargos are selectively recognized and enclosed by autophagosomal membranes are still unknown.
Prof. ZHANG Hong’s group investigated structure/function relationships of the two C. elegans Atg8 homologs, LGG-1 and LGG-2. The former is essential for degradation of a variety of protein aggregates, while the latter has cargo-specific and developmental stage-specific roles.
LGG-1 and LGG-2 interact differentially with autophagy substrates and Atg proteins. Differential interaction of LGG-1 and LGG-2 with different Atg proteins ensures the ordered recruitment of Atg proteins, which allows them to function at different steps of the autophagy pathway.
LGG-1 and LGG-2 possess two hydrophobic pockets, known as the W-site and the L-site, through which the LIR motif present in Atg8-binding proteins can be recognized. However, LGG-1 and LGG-2 have structurally distinct W- and L-sites that exhibit different preferences for residues in the LIR motif. The N-terminal tails of LGG-1 and LGG-2 adopt unique closed and open conformations, respectively, which may result in distinct membrane tethering and fusion activities.
The differential and non-redundant function of LGG-1 and LGG-2 in autophagy provides insights into the distinct function of GABARAP and LC3 subfamily members in autophagy in mammalian cells. It has been largely assumed that autophagy genes are equally employed for removal of different substrates.
However, this study demonstrates that an autophagy gene can exhibit temporal-specific and cargo-specific functions to accommodate the characteristics of different types of cargo and distinct developmental stages during multicellular organism development.
The study was supported by grants from the NSFC, the National Basic Research Program of China, and in part by an International Early Career Scientist grant from the Howard Hughes Medical Institute; Dr. Nobuo N. Noda (another corresponding author) was supported by Grants-in-Aid for Scientific Research on Priority Areas.
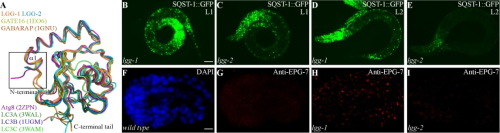
Figure legend: LGG-1 and LGG-2 show structural differences and lgg-2 exhibits temporal-specific and cargo-specific functions in aggrephagy. (Image by IBP)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

