
Newsroom
A research team led by Prof. HU Linhua from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences has developed a highly durable hydrogel electrolyte for aqueous zinc-ion batteries (AZIBs) by using urea as a zincophilic solubilizer and zinc acetate (Zn(Ac)₂) salt—an affordable and environmentally friendly material.
Their findings were recently published in Angewandte Chemie International Edition.
The newly designed hydrogels can sustain 557% tensile elongation and 3.7 MPa compressive strength. In-situ polyurea solid electrolyte interphase (SEI) formed during AZIB operation enables stable Zn stripping/plating in a dendrite and passivation-free manner.
"This approach overcomes the usual limits of the low-cost Zn(Ac)2 salt, making it much better at resisting wear and tear," said LI ZHAO Qian, a member of the team, "it allows the material to withstand repeated processes of zinc plating and stripping, as well as other physical stress, improving its overall durability."
Aqueous zinc-ion batteries have long faced challenges including electrolyte leakage and electrode corrosion. While quasi-solid-state electrolytes provide better stability and flexibility, they often fall short in cost-effectiveness, environmental friendliness, and fatigue resistance. Zinc acetate is attractive due to its low cost and eco-friendly nature but suffers from poor solubility, restricting battery capacity and performance.
To address this, the researchers employed a novel strategy leveraging the "salting out" effect, which increases zinc acetate solubility by removing hydration layers around polymer chains, thereby strengthening their network. This enhancement boosts fatigue resistance, allowing the electrolyte to better withstand repeated electrochemical cycling and external mechanical deformation.
During battery operation, a protective layer naturally forms on the electrode, improving the overall stability of the battery's interface. The zinc-ion battery shows excellent efficiency, and the flexible pouch cell performs well in terms of capacity and stability, even after many cycles. The flexibility of the pouch cell is particularly notable, as it can maintain a steady voltage even when bent or folded, making it suitable for use in portable and wearable devices.
"When the flexible pouch battery is subjected to varying degrees of bending, it still retained a stable voltage even at 180°. These findings highlight its potential for application in portable and wearable electronic devices," said Dr. LI Zhaoqian.
The researchers also evaluated the battery's performance in terms of rate capability and self-discharge. The full Zn//NH4V4O10 battery with the USPH-5 electrolyte showed excellent capacity, even after repeated cycling. After a long rest period, it still delivered a strong discharge capacity, while batteries without the USPH-5 electrolyte showed a significant loss in capacity. This demonstrates that the new electrolyte material greatly enhances the battery's overall performance and retention over time.
This study not only highlights the zincophilic solubilization to break salt solubility limit in quasi-solid-state AZIBS, but also provides an extendable regulation strategy for other metal anodes to fulfill low cost, eco-friendly and high-performance batteries.
This work was supported by the HFIPS Director's Fund, the Anhui Provincial Natural Science Foundation and the Anhui Provincial Science and Technology Innovation Initiative.
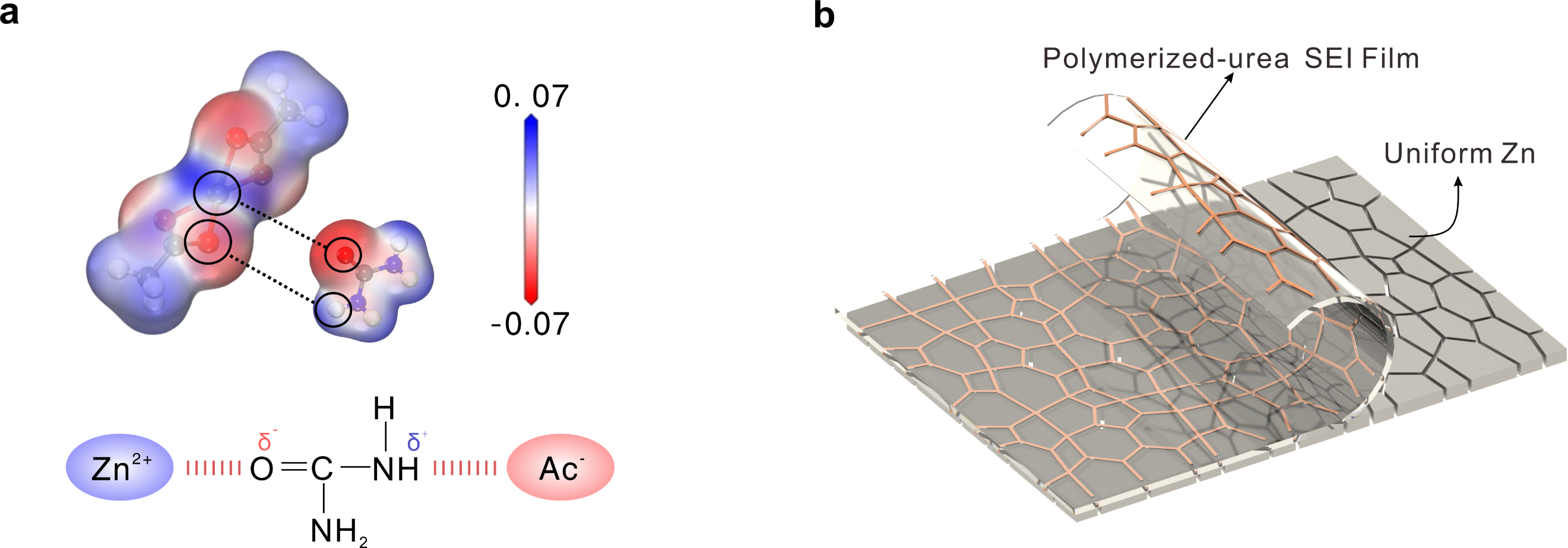
Figure 1. (a) Calculated electrostatic potential (ESP) distribution of Zn(Ac)2 and urea, and schematic representation of the Zn2+-urea-Ac- system. (b) Schematic illustration about the uniform Zn deposition by using the USPH-5. (Image by LI Zhaoqian)
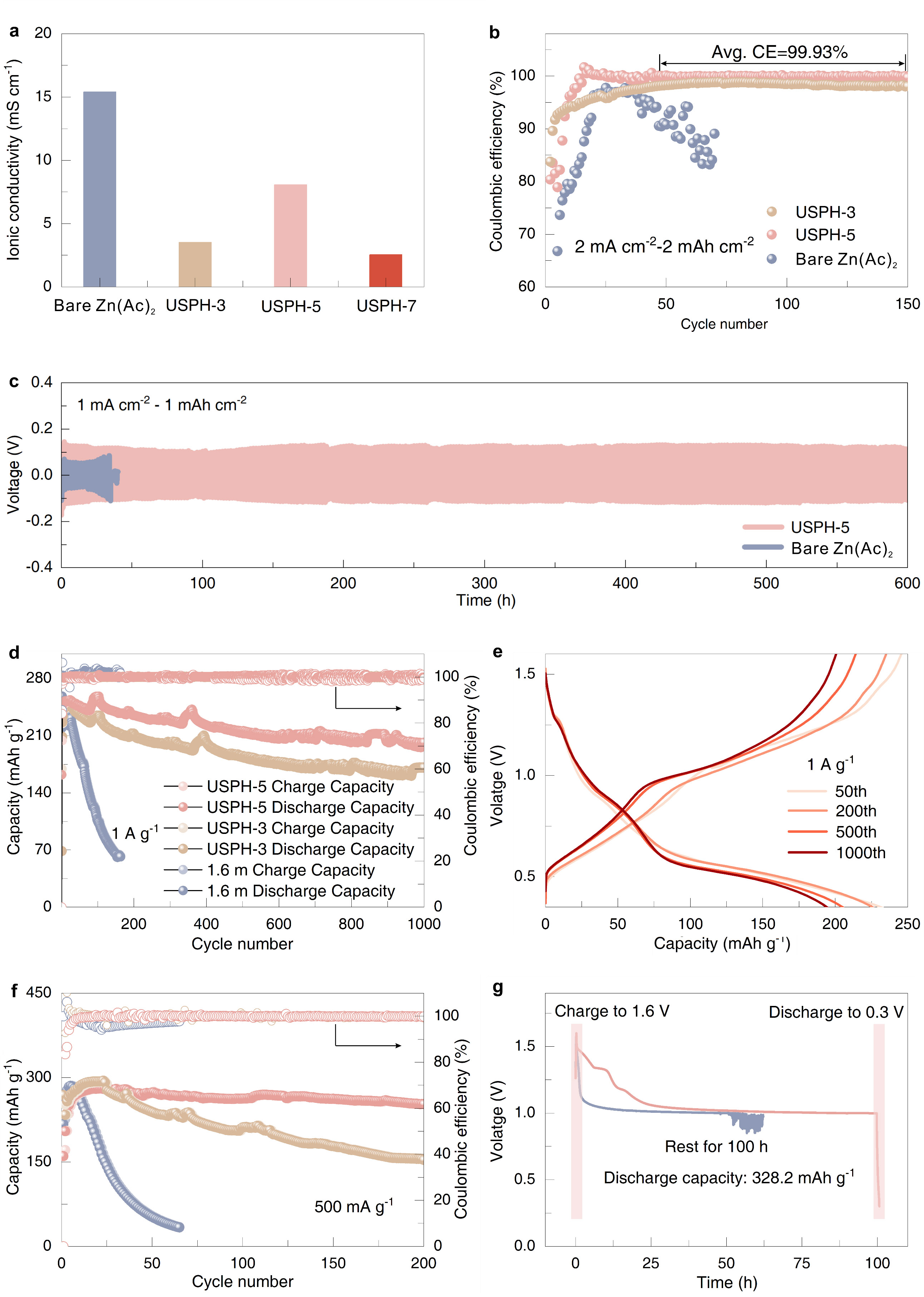
The properties comparison of different electrolytes (Image by LI Zhaoqian)
