
Scientists from the Institute of Biophysics of the Chinese Academy of Sciences reported the molecular basis of self-assembly and compositional activation of Latent Membrane Protein 1 (LMP1). They discovered that LMP1 undergoes oligomeric self-assembly through a novel mechanism, which is different from previous speculations, and that recruits downstream factors in an efficient way to activate and maintain the activation of pathogenic signals.
This study was published in Cell on July 11, 2024.
Epstein-Barr Virus (EBV) is a human herpesvirus that poses a significant threat to human health. LMP1 is a key oncogenic protein encoded by EBV and has long been considered an ideal target for differential diagnosis and targeted therapy of EBV-positive tumors. How LMP1 achieves ligand-independent assembly and activation remains a fundamental open question as well as a critical obstacle to the successful development of LMP1-targeted intervention strategies.
In the study, the researchers successfully resolved cryo-EM structures of LMP1 in two unexpected assemblies: a symmetric homodimer and a higher-order filamentous oligomer. LMP1 adopts a non-canonical and unpredicted fold that supports the formation of a stable homodimer through tight and antiparallel inter-molecular stacking. The LMP1 dimer serves as the basic unit for higher-order assembly, with multiple dimers self-assembling in a side-by-side manner to form filamentous oligomeric structures.
Importantly, these high-resolution structural insights were supported by the observation of unique aggregation patterns of LMP1 observed in live-cell imaging, revealing the membrane clustering mechanism of LMP1 at different resolution scales.
To further clarify the functional form of LMP1, the researchers systematically mutated the interfaces of dimers and oligomers. The results confirmed that the oligomeric fibrous structure of LMP1 represents its activated state.
Through careful analysis, the researchers discovered that the filamentous assembly of LMP1 arranges an approximately equilateral triangular distribution for the cytoplasmic tails of adjacent LMP1 dimers, further facilitating recognition between the symmetric TRAF trimer and LMP1. The ligand-independent self-association ability of LMP1 molecules and their large number in each higher-order oligomer allow LMP1 to induce potent downstream signaling processes in a constitutive and ligand-independent manner.
This work addresses a long-standing challenge in the field, and the newly discovered mechanism and interface hold the promise for future development of LMP1-targeted intervention strategies.
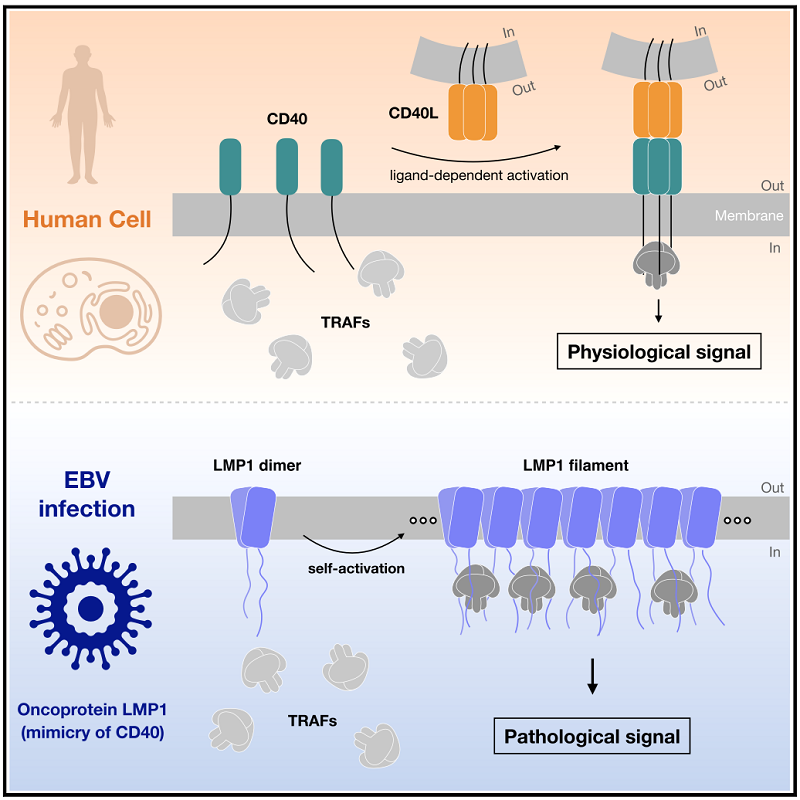
Different Activation Mechanisms of CD40 and LMP1. (Image by GAO Pu's group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

