
A research team led by Dr. LI Zhaoqian from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences found that the addition of 1,4-dioxane (DX) molecules to the electrolyte of aqueous zinc-ion (Zn) battery would lead to the growth of the preferred Zn (002) texture, effectively suppressing the Zn dendrite growth and improving the reversibility and cycling stability of the batteries.
The study was published in ACS Nano.
Aqueous rechargeable zinc-ion batteries (ZIBs) are an emerging sustainable system for the next generation of grid-scale energy storage technology. However, the implementation of this technology has been plagued by the severe dendrite problem and poor reversibility of the Zn anode.
In the hexagonal close-packed Zn crystal, the (002) facet has the lowest surface energy and the slowest growth rate, which enables a surface-reaction-controlled deposition process and thus mitigates the rampant Zn2+ flux and side-reactions. Therefore, inducing a preferred Zn (002) texture can effectively alleviate the dendrite growth and the formation of side-reactions.
In this study, the researchers constructed an advanced electrolyte modulation strategy to adjust the anode/electrolyte interface. In this new system, the adsorption of DX on the Zn surface can induce Zn (002) texture growth and suppress the detrimental side-reactions.
The performance of the new system was validated in subsequent experiments. The battery with the addition of DX showed a long-term cycling stability of 1000 h, even under the harsh condition of 10 mA cm-2 with an ultra-high cumulative plated capacity of 5 Ah cm-2. The battery also showed a high reversibility with an average coulombic efficiency of 99.7%.
"The Zn//NH4V4O10 full cell with DX realized high specific capacity and capacity retention," said Dr. LI Zhaoqian, "it is much better than ZIBs with pure ZnSO4 electrolyte."
This study, which selectively adjusts the deposition rate of Zn2+ on the crystal plane by adsorbing molecules, provides a promising strategy for modulating high-performance zinc anodes at the molecular level and is expected to be applicable to other metal anodes with poor stability and reversibility.
This study was supported by the HFIPS Director's Fund.
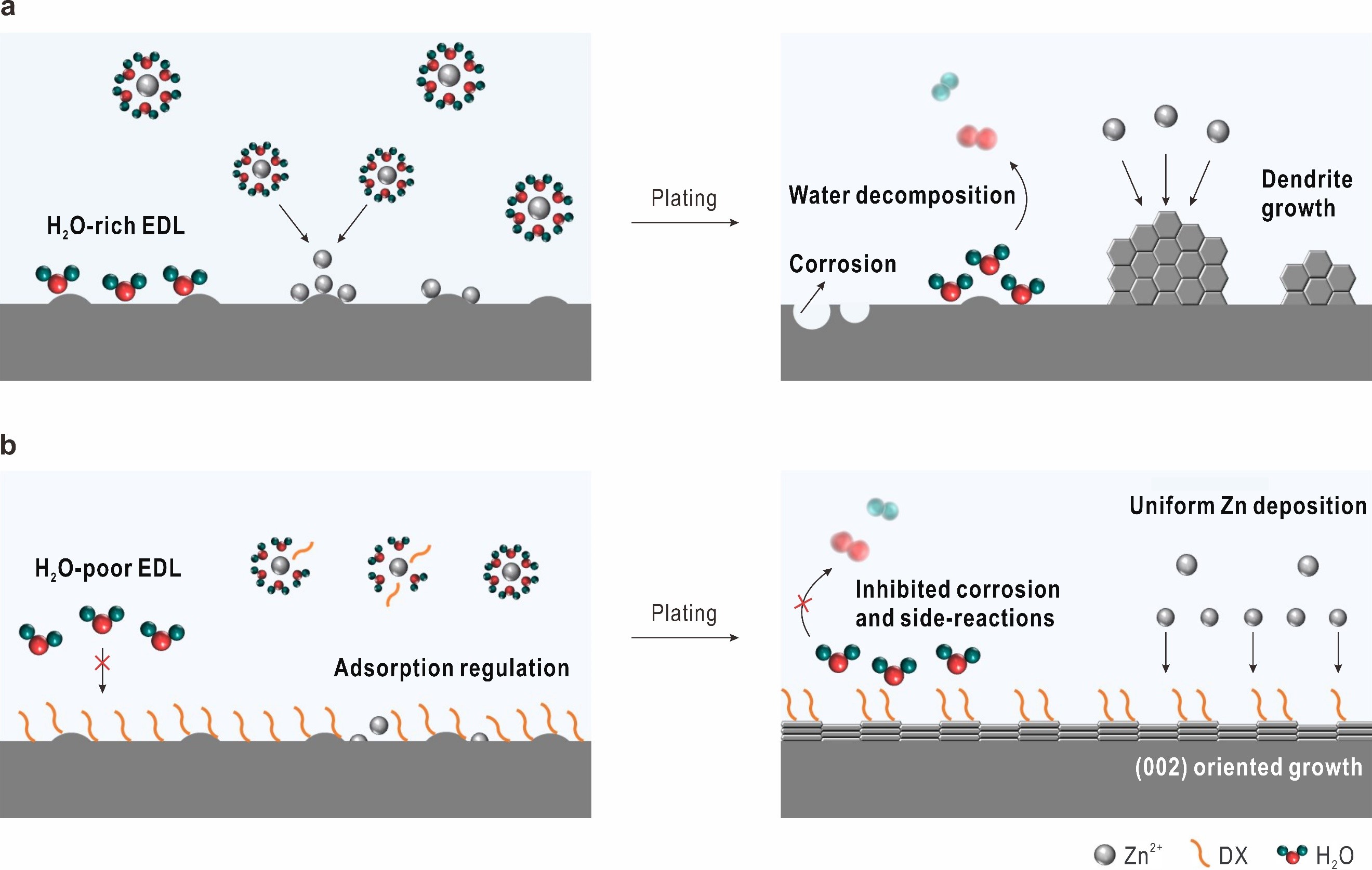
The schematic diagrams for Zn deposition cycled in different electrolytes. a. bare ZnSO4 electrolyte; b. DX/ZnSO4 electrolyte. (Image by LI Zhaoqian)

a. The in-situ optical microscopic comparison of the deposited Zn anode surface in the ZnSO4 electrolyte and DX/ZnSO4 electrolyte; b, c. The XRD patterns and SEM images show the (002) texture in the DX/ZnSO4 electrolyte; d, e. The adsorption energy comparison. (Image by LI Zhaoqian)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

