
According to a recent study published in Advanced Science, scientists have successfully improved the specific capacity of Na3V2(PO4)3 (NVP), a new electrode material. They also found its relatively exotic extrinsic pseudocapacitance behavior.
This work, conducted by a research group led by Prof. ZHAO Bangchuan from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences, effectively activates the M1 site in the structure of NVP by reducing the crystallinity of the material, and realizes the reversible insertion and removal of three sodium ions.
NVP has a hexagonal NASICON (or sodium super ion conductor) structure. Sodium ions occupy two unequal Wyckoff sites, 1/3 of which are located at 6b site (M1) and 2/3 at 18e site (M2). From the point of view of thermodynamic equilibrium and kinetics, sodium ions at M1 site are difficult to participate in the redox reaction. There is no electrochemical reactivity during the charging and discharging processes. The reversible insertion and removal of sodium ions only occurs at M2 site. Only two sodium ions participate in the electrochemical reaction through the V4+/V3+redox pair. Therefore, the theoretical specific capacity of NVP is just 117.6 mAh g-1. Whether the specific capacity of NVP can be improved by activating M1 site and using V5+/V4+redox reaction is a great challenge in this field.
In this work, NVP precursor was deposited on carbon foam substrate by electrostatic spray method, and the crystallinity of NVP was adjusted by controlling the annealing temperature. Two NVP materials, NVP-E600 and NVP-E700, were obtained. The corresponding annealing temperatures were 600 and 700 °C, respectively.
With nanocrystalline and amorphous phase coexisting structure NVP material as the cathode and the metal sodium sheet as the counter electrode, a coin-type cell was assembled, and its sodium storage performance was evaluated.
The results showed that the nanocrystalline and amorphous phase coexisting structure NVP material had excellent rate performance and cycle stability due to the reversible de-insertion of three sodium ions. It displayed a specific capacity of 179.6 mAh g-1 at 0.2 C (1 C = 117.6 mA g-1), and the capacity retention rate is 99.6% after 200 cycles. Even at a high rate of 10 C, it also has a specific capacity of 73.5 mAh g-1.
The test results of electrochemical impedance spectrum and cyclic voltammetry curves show that this disordered NVP material has strong electrochemical reaction kinetics, and the charge storage was mainly pseudocapacitive, which is very different from the crystalline NVP with battery-type charge storage behavior.
The extrinsic pseudocapacitance behavior in the highly disordered material can be attributed to the fact that the introduction of disordered structure changes the interaction between sodium ions in NVP materials, which makes the charge-discharge process change from the original two-phase reaction to single-phase reaction. This causes the disappearance of the platform in the charge-discharge curves and the rectangle of the cyclic voltammetry curves.
This demonstrates the importance of the disordered structure for the three-electron reaction process and extrinsic pseudocapacitance in NVP cathodes of sodium-ion batteries, according to the team.

Origin of three-electron reaction mechanism and extrinsic pseudocapacitance behavior for the new NVP material. (Image by MA Hongyang)
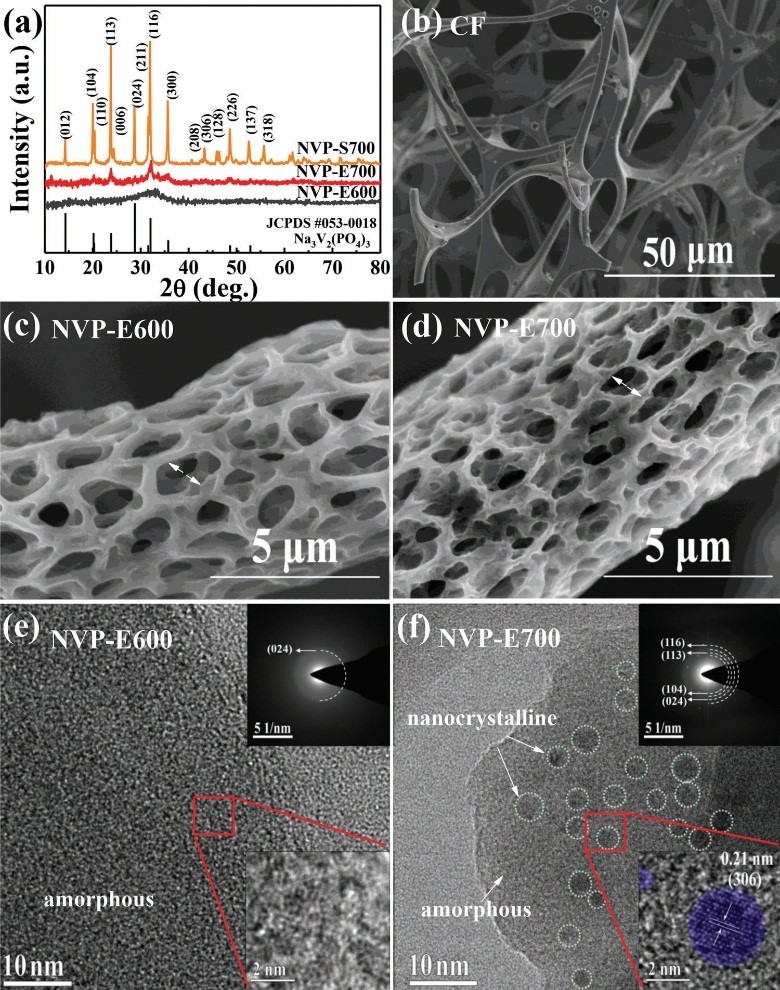
Structure and morphology characterization results of NVP materials with different crystallinity. (Image by MA Hongyang)
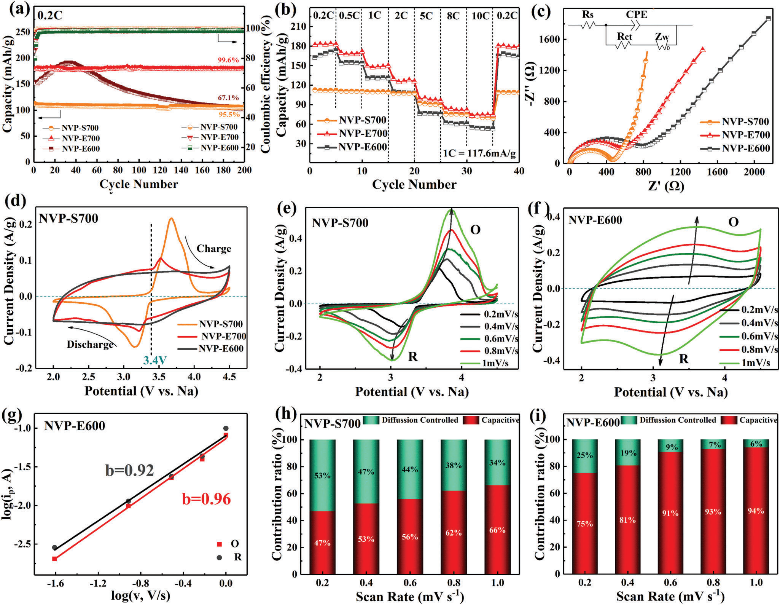
Electrochemical performance measurements of NVP material with nanocrystalline and amorphous phase coexistence structure. (Image by MA Hongyang)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

