
Newsroom
Hydrodeoxygenation is a common way to reduce the oxygen content of biomass fuel. Compared with direct hydrogenation, transfer hydrogenation is safer and cheaper, as it uses a protic solvent as hydrogen source, thus avoiding the use of high pressurized hydrogen.
Recently, a team led by Prof. CHEN Chun from the Institute of Solid State Physics of the Hefei Institute of Physical Science (HFIPS) displayed the effect of strong metal-support interaction (SMSI) on catalytic performance.
They synthesized Cu/SiO2 and Cu/CeO2 catalysts respectively, and studied the effects of the SMSI effect between copper and metal oxide support or inert support on the hydrogenation of unsaturated aldehydes.
At first, the team used silica spheres as precursors to synthesize a sea urchin-like Cu0/Cu doped SiO2 catalyst, and due to the SMSI effect between Cu and SiO2 support, Cu exists in the form of sub-nano clusters and single atoms on SiO2 surface. The catalyst showed excellent activity in the transfer hydrogenation of vanillin to 2-methoxy-4-methylphenol (MMP). The complete conversion of vanillin and 100% yield of MMP were reached under the condition of 140 ℃, and isopropanol was used as the reaction solvent and hydrogen source.
They also studied the effect of the Cu-SiO2 interface on the catalytic reaction by Density Functional Thoery (DFT) calculation.
This preliminary study has important guiding significance for the preparation of highly efficient non noble metal catalysts by chemical template method and have potential industrial application prospects.
As the research in the liquid-phase catalytic reaction is still insufficient, they conducted another research on Cu/CeO2, and the effects of CeO2 crystal surface on Cu and liquid-phase hydrogenation. They synthesized CeO2 with different morphologies and crystal faces.
Results showed that Cu/CeO2-P (copper loaded on nano-polyhedral CeO2 with (1 1 1) terminated surface) has the best performance, it could achieve the complete conversion of vanillin at 130 ℃, while Cu/CeO2-R (Cu loaded on nano-rod CeO2 with (1 1 0) plane) and Cu/CeO2-C (Cu loaded on nano-cube CeO2 with (1 0 0) facet) can convert 50% and 10% vanillin respectively under the same conditions. This difference is due to the weakest SMSI effect between (111) crystal surface exposed by CeO2-P and Cu so that Cu on the surface is easier to be reduced to Cu0 with high activity.
As a result, the surface of Cu/CeO2-P has more active sites, showing the best reaction activity.
"For different systems, sometimes too strong SMSI effect can inhibit the reaction activity of active metals," said CHEN Chun from this research, "which has a negative impact on the catalytic activity."
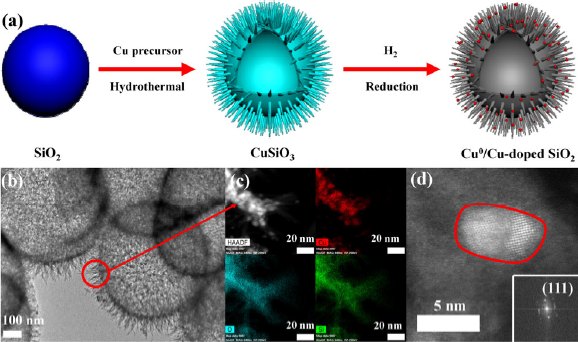
Fig. 1. (a) Synthesis scheme, (b-d) Morphology of Cu0/Cu-doped SiO2 catalysts (Image by CHENG Chun)
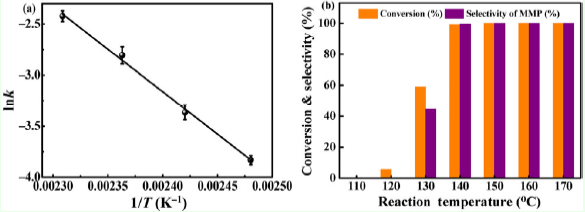
Fig. 2. Catalytic performance of Cu0/Cu-doped SiO2 catalysts. (Image by CHENG Chun)
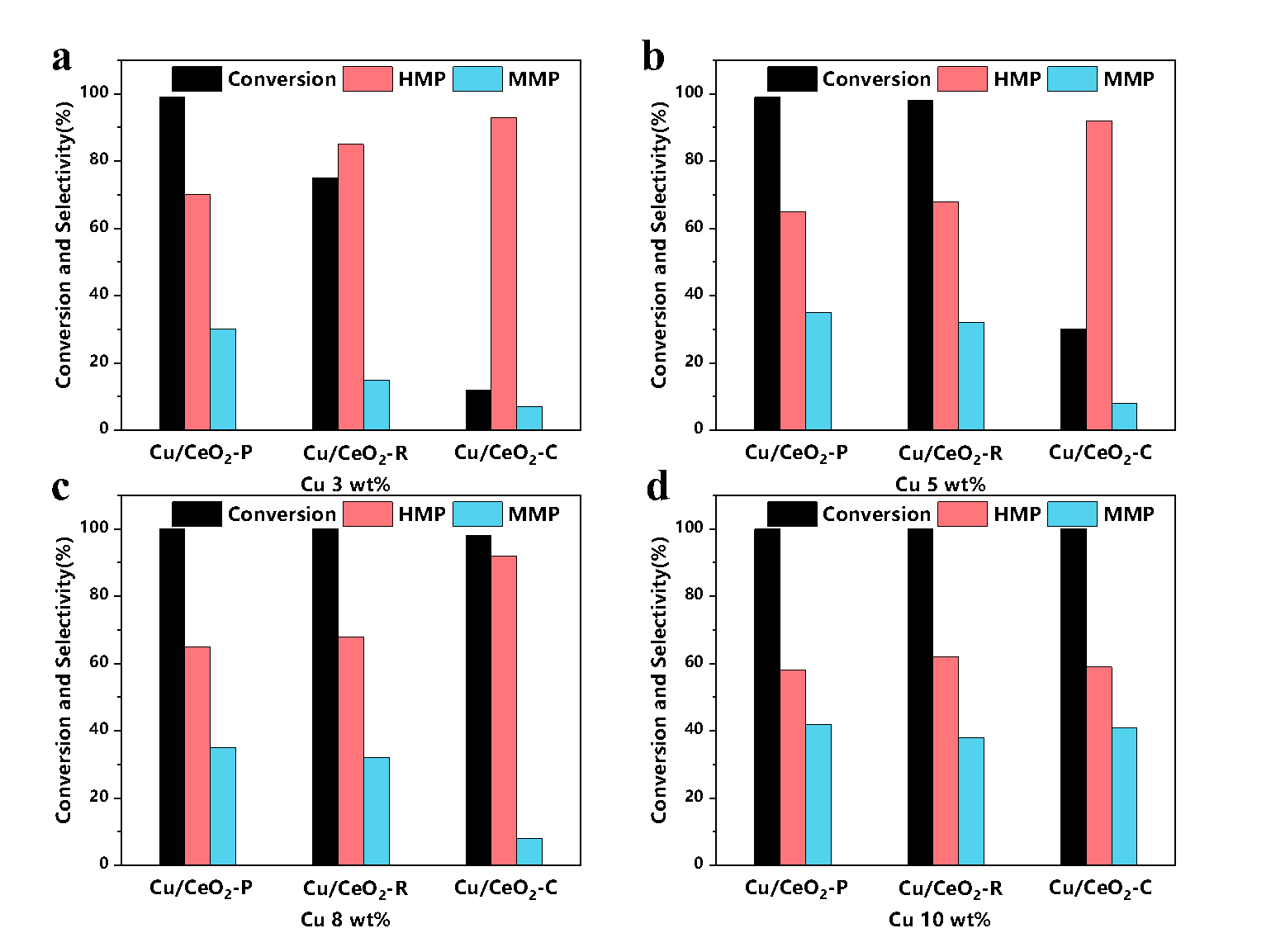
Fig. 3. Catalytic performance of Cu/CeO2 catalysts (Image by CHENG Chun)
