
Newsroom
O-linked β-N-acetylglucosamine (O-GlcNAc) is a reversible and ubiquitous post-translational modification in eukaryotic cells, it involves in embryonic development or fetal health, whereas the underlying mechanisms remain unclear.
The placenta is a temporary and indispensable organ that is responsible for the intrauterine development of the fetus during pregnancy. The syncytium layer is at the outermost of the placenta, it contains multinucleated syncytotrophoblast (STB) that is formed by the cell fusion of monocytotrophoblasts (CTB), a process of syncytosis caused mainly by the activation of protein kinase A (PKA). STB is directly bathed in maternal blood to regulate feto-maternal material exchange. Revealing the regulatory mechanisms of placental trophoblast differentiation is of great value for maternal-fetal health. To date, the dynamic property and working mechanism of O-GlcNAcylation in trophoblast syncytialization have not been thoroughly studied.
A research group led by Prof. WANG Yanling at the Institute of Zoology of the Chinese Academy of Sciences (CAS), in collaboration with Drs. WANG Chu and CHEN Xing from Peking University, has now made a major breakthrough about the regulation of trophoblast differentiation by post-translational protein modification through applying the quantitative O-GlcNAc proteomics in human trophoblasts. This study was published in Cell Chemical Biology on Feb. 23, 2021.
The researchers employed the chemoenzymatic O-GlcNAc labeling and the reductive dimethylation-based quantitative proteomics to quantify the dynamics of O-GlcNAcylated proteins during in vitro syncytialization of BeWo cells (a human trophoblastic cell line) induced by PKA activator, forskolin.
They established the first global dataset of O-GlcNAcylated proteins from human placental trophoblast cell, and hundreds proteins that were dynamically O-GlcNAcylated during trophoblast syncytialization were identified.
Among those PKA-induced GlcNAcylated proteins, Cystathionine γ-lyase (CSE) exhibited the most significant change. By applying the methods of click chemistry and quantitative O-GlcNAc stoichiometry, it was revealed that CSE O-GlcNAcylation in trophoblasts was a very early response to PKA and exerted steady-going influence on CSE function.
Site-specific analysis by mass spectrometry revealed Ser138 as the core O-GlcNAc site in CSE, and its O-GlcNAcylation promoted the enzymatic activity of CSE to produce H2S. The GlcNAcylated CSE-boosted H2S efficiently inhibited androgen receptor (AR) dimerization and thus hampered testosterone-enhanced trophoblast syncytialization.
The multidisciplinary approach in this study provides a resource of O-GlcNAc dynamics in human placenta and uncovers a key role of CSE O-GlcNAcylation in controlling trophoblast differentiation. The finding reveals a guarding mechanism CSE O-GlcNAcylation to control an appropriate degree of syncytialization through coordinating PKA and AR signals.
The study make a deeper insight into the biological significance of O-GlcNAcylation in placental development as well as the potential therapeutic targets for the placenta-associated pregnant complications.
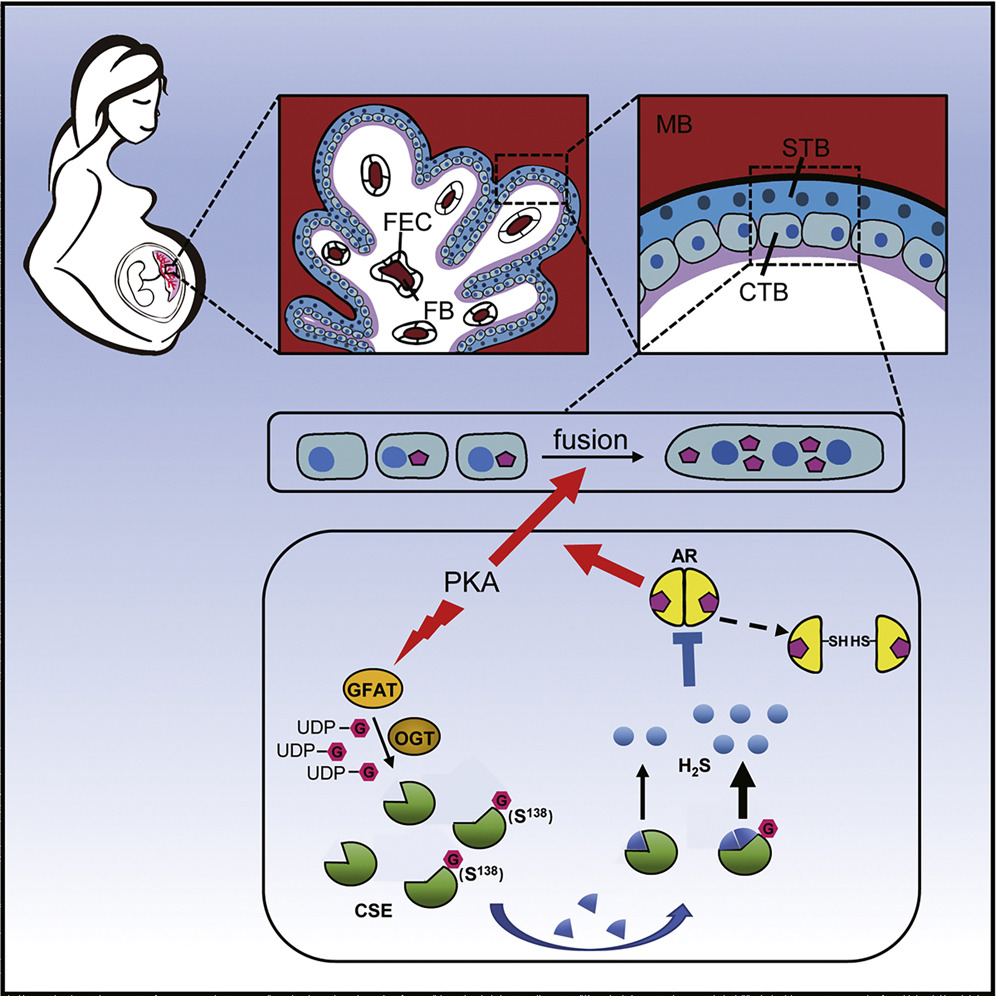
The working model of cystathionine γ-lyase (CSE) O-GlcNAcylation to modulate trophoblast syncytialization. (Image by IOZ)
